TFAM 是一种自噬受体,它通过与细胞质线粒体 DNA 结合来限制炎症的发生
IF 17.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
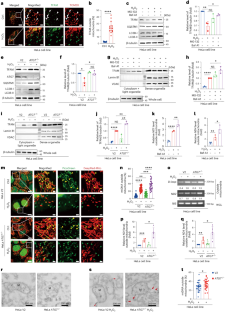
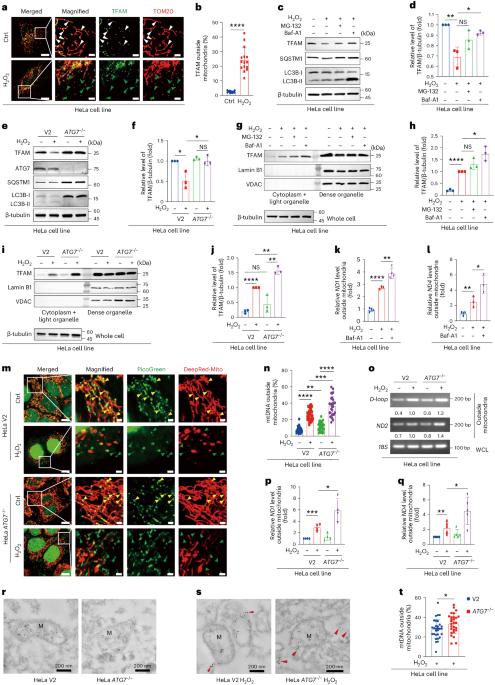
当细胞受到压力时,产生能量的线粒体中的 DNA 如果不被清除,就会泄漏出来,引发炎症免疫反应。细胞采用一种名为自噬的质量控制系统来专门降解受损成分。我们发现,线粒体转录因子A(TFAM)--一种与线粒体DNA(mtDNA)结合的蛋白质--通过自溶酶体途径与自噬蛋白LC3相互作用(我们称之为核吞噬),有助于消除泄漏的mtDNA。TFAM 含有一个被称为 LC3 相互作用区(LIR)的分子代码,可实现这种结合。虽然突变 TFAM 的 LIR motif 不会影响其正常的线粒体功能,但细胞胞质中积累了更多的 mtDNA,激活了炎症信号通路。因此,TFAM介导自噬清除泄漏的mtDNA,从而限制炎症。确定这一机制有助于进一步了解细胞如何利用自噬机制选择性地靶向和降解炎性mtDNA。这些发现可为涉及线粒体损伤和炎症的疾病研究提供参考。本文章由计算机程序翻译,如有差异,请以英文原文为准。
TFAM is an autophagy receptor that limits inflammation by binding to cytoplasmic mitochondrial DNA
When cells are stressed, DNA from energy-producing mitochondria can leak out and drive inflammatory immune responses if not cleared. Cells employ a quality control system called autophagy to specifically degrade damaged components. We discovered that mitochondrial transcription factor A (TFAM)—a protein that binds mitochondrial DNA (mtDNA)—helps to eliminate leaked mtDNA by interacting with the autophagy protein LC3 through an autolysosomal pathway (we term this nucleoid-phagy). TFAM contains a molecular zip code called the LC3 interacting region (LIR) motif that enables this binding. Although mutating TFAM’s LIR motif did not affect its normal mitochondrial functions, more mtDNA accumulated in the cell cytoplasm, activating inflammatory signalling pathways. Thus, TFAM mediates autophagic removal of leaked mtDNA to restrict inflammation. Identifying this mechanism advances understanding of how cells exploit autophagy machinery to selectively target and degrade inflammatory mtDNA. These findings could inform research on diseases involving mitochondrial damage and inflammation. Liu, Zhen, Xie, Luo, Zeng, Zhao et al. show that the major nucleoid protein TFAM interacts with cytoplasmic LC3B during oxidative or inflammatory stress to attenuate mitochondrial DNA-induced inflammation via the cGAS–STING pathway.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


