食用菌中吡咯和呋喃酮衍生物的分离、绝对构型和免疫抑制活性
IF 1.3
4区 生物学
Q4 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
通过对灰口草子实体甲醇提取物的化学研究,分离出了七种吡咯生物碱和两种呋喃酮代谢物,其中包括两种未曾描述过的代谢物(化合物 1 和 3)。这些化合物的结构是通过光谱数据分析和量子化学计算确定的。根据其光学旋转和电子圆二色性,并在量子计算的帮助下,首次确定了 2 的绝对构型。化合物 1 对 LPS 诱导的 B 淋巴细胞和 Con A 诱导的 T 淋巴细胞的细胞增殖具有抑制活性,前者的 IC50 值分别为 30.53 μM,后者的 IC50 值为 46.79 μM。本文章由计算机程序翻译,如有差异,请以英文原文为准。
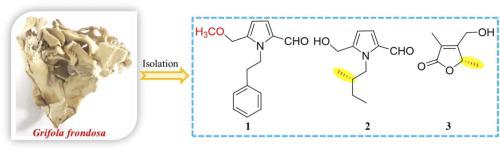
Isolation, absolute configuration and immunosuppressant activities of pyrrole and furanone derivatives from the edible mushroom Grifola frondosa
Chemical investigation on methanol extract of the fruiting bodies of Grifola frondosa led to the isolation of seven pyrrole alkaloids and two furanone metabolites, including two undescribed ones (compounds 1 and 3). The structures were established by spectroscopic data analysis and quantum chemical calculations. The absolute configuration of 2 was established for the first time on the basis of its optical rotation and electronic circular dichroism aided with quantum calculations. Compound 1 showed the suppressive activity against the cell proliferation of LPS-induced B lymphocytes with IC50 value of 30.53 μM and Con A-induced T lymphocytes with IC50 value of 46.79 μM, respectively.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytochemistry Letters
生物-生化与分子生物学
CiteScore
3.00
自引率
11.80%
发文量
190
审稿时长
34 days
期刊介绍:
Phytochemistry Letters invites rapid communications on all aspects of natural product research including:
• Structural elucidation of natural products
• Analytical evaluation of herbal medicines
• Clinical efficacy, safety and pharmacovigilance of herbal medicines
• Natural product biosynthesis
• Natural product synthesis and chemical modification
• Natural product metabolism
• Chemical ecology
• Biotechnology
• Bioassay-guided isolation
• Pharmacognosy
• Pharmacology of natural products
• Metabolomics
• Ethnobotany and traditional usage
• Genetics of natural products
Manuscripts that detail the isolation of just one new compound are not substantial enough to be sent out of review and are out of scope. Furthermore, where pharmacology has been performed on one new compound to increase the amount of novel data, the pharmacology must be substantial and/or related to the medicinal use of the producing organism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


