以原子分辨率成像冰 Ih 的表面结构和预熔化过程
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
冰表面与许多物理和化学特性密切相关,如熔化、冻结、摩擦、气体吸收和大气反应1,2,3,4,5,6,7,8。尽管进行了广泛的实验和理论研究9,10,11,12,13,14,15,16,17,但由于氢键网络的脆弱性和复杂的预熔化过程,冰界面的确切原子结构仍然难以捉摸。在这里,我们利用基于 qPlus 的低温原子力显微镜和一氧化碳功能化针尖,实现了六方水冰(冰 Ih)基底(0001)表面结构的原子分辨率成像。我们发现结晶冰-Ih 表面由混合的 Ih- 和立方(Ic)-堆积纳米域组成,形成了 (\sqrt{19}\times \sqrt{19}\)周期性超结构。密度泛函理论显示,这种重构表面比理想的冰表面更稳定,主要是通过最大限度地减少悬空 OH 键之间的静电排斥。此外,我们还观察到,随着温度的升高(超过 120 开尔文),冰表面逐渐变得无序,这表明预熔化过程已经开始。表面预熔化发生在 Ih 和 Ic 结构域之间的缺陷边界上,平面局部结构的形成可以促进表面预熔化。这些结果结束了关于冰表面结构的长期争论,揭示了冰预熔化的分子起源,可能会导致对冰物理和化学认识的范式转变。本文章由计算机程序翻译,如有差异,请以英文原文为准。
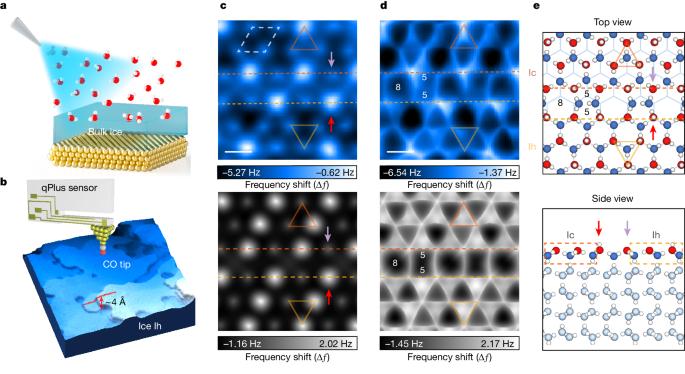
Imaging surface structure and premelting of ice Ih with atomic resolution
Ice surfaces are closely relevant to many physical and chemical properties, such as melting, freezing, friction, gas uptake and atmospheric reaction1–8. Despite extensive experimental and theoretical investigations9–17, the exact atomic structures of ice interfaces remain elusive owing to the vulnerable hydrogen-bonding network and the complicated premelting process. Here we realize atomic-resolution imaging of the basal (0001) surface structure of hexagonal water ice (ice Ih) by using qPlus-based cryogenic atomic force microscopy with a carbon monoxide-functionalized tip. We find that the crystalline ice-Ih surface consists of mixed Ih- and cubic (Ic)-stacking nanodomains, forming $$\sqrt{19}\times \sqrt{19}$$ periodic superstructures. Density functional theory reveals that this reconstructed surface is stabilized over the ideal ice surface mainly by minimizing the electrostatic repulsion between dangling OH bonds. Moreover, we observe that the ice surface gradually becomes disordered with increasing temperature (above 120 Kelvin), indicating the onset of the premelting process. The surface premelting occurs from the defective boundaries between the Ih and Ic domains and can be promoted by the formation of a planar local structure. These results put an end to the longstanding debate on ice surface structures and shed light on the molecular origin of ice premelting, which may lead to a paradigm shift in the understanding of ice physics and chemistry. Atomic-resolution imaging of the surface structure of hexagonal water ice is achieved using cryogenic atomic force microscopy, providing a molecular perspective on the origin and mechanism of of ice premelting.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


