二苯甲酮在光诱导 C(sp3)-H 单氟烯化反应中的二合一作用:氢原子转移和单电子转移。
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
该研究报告提出了一种利用二氟烯烃对 C(sp3)-H键进行单氟烯化反应的新方案。在该方案中,二苯甲酮作为光催化剂具有氢原子转移和单电子转移的双重作用。二苯甲酮的激发态会从 C(sp3)-H 键中抽取一个氢原子,生成相应的碳自由基,碳自由基随后会发生自由基加成/SET/β-F 消去过程,而不是之前工作中的自由基-自由基交叉偶联。该反应对醚、硫醚和胺的α碳原子具有很高的区域选择性,从而能够制备单氟烯。本文章由计算机程序翻译,如有差异,请以英文原文为准。
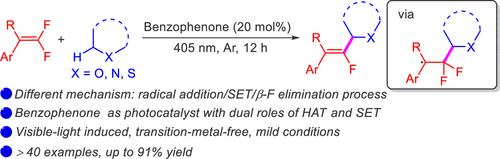
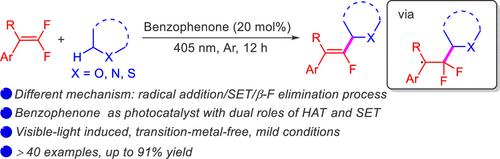
Two-in-One Role of Benzophenone in Photoinduced C(sp3)–H Monofluoralkenylation: Hydrogen-Atom Transfer and Single-Electron Transfer
A new protocol for monofluoralkenylation of C(sp3)–H bonds with gem-difluoroalkenes was reported. In this protocol, benzophenone serves as a photocatalyst with dual roles of hydrogen-atom transfer and single-electron transfer. The excited state of benzophenone abstracts a hydrogen atom from a C(sp3)–H bond to generate a corresponding carbon radical, which subsequently undergoes a radical addition/SET/β-F elimination process rather than radical–radical cross-coupling of previous work. This reaction shows high regioselectivity for the α-carbon atoms of ethers, thioethers, and amines, enabling the preparation of monofluoroalkenes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


