RNAi 筛选发现 HES4 是支持嘧啶合成和肿瘤生长的氧化还原平衡调节器
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
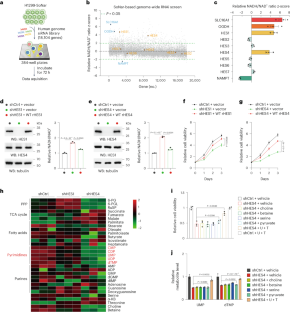
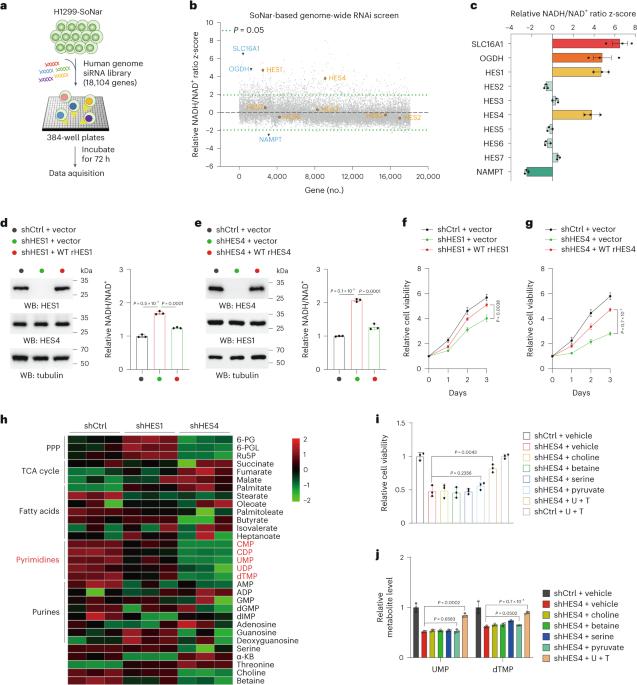
NADH/NAD+ 氧化还原平衡对细胞代谢至关重要。尽管目前缺乏对 NAD(H)氧化还原调节因子的系统鉴定,但这将有助于发现与协调生长代谢密切相关的未知效应因子。在这项研究中,我们进行了基因组规模的 RNA 干扰(RNAi)筛选,以全面调查参与氧化还原调节的基因,并发现 HES 家族 bHLH 转录因子 HES4 是 NADH/NAD+ 比率的负调控因子。从功能上看,HES4 对维持线粒体电子传递链(ETC)活性和嘧啶合成至关重要。更具体地说,HES4 直接抑制 SLC44A2 和 SDS 的转录,从而分别抑制线粒体胆碱氧化和细胞膜丝氨酸脱氨,进而确保辅酶 Q 还原能力,以促进 DHODH 介导的 UMP 合成和丝氨酸衍生的 dTMP 生成。因此,抑制胆碱氧化可保持线粒体丝氨酸分解代谢和 ETC 耦合氧化还原平衡。此外,在表皮生长因子受体(EGFR)激活的情况下,HES4 蛋白的稳定性会增强,HES4 水平的增加会促进 EGFR 驱动的肿瘤生长,并预测肺腺癌的不良预后。这些发现说明了丝氨酸和胆碱分解代谢之间的嘧啶生物合成的未知机制,并强调了 HES4 在肿瘤代谢中的生理重要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
RNAi screens identify HES4 as a regulator of redox balance supporting pyrimidine synthesis and tumor growth
NADH/NAD+ redox balance is pivotal for cellular metabolism. Systematic identification of NAD(H) redox regulators, although currently lacking, would help uncover unknown effectors critically implicated in the coordination of growth metabolism. In this study, we performed a genome-scale RNA interference (RNAi) screen to globally survey the genes involved in redox modulation and identified the HES family bHLH transcription factor HES4 as a negative regulator of NADH/NAD+ ratio. Functionally, HES4 is shown to be crucial for maintaining mitochondrial electron transport chain (ETC) activity and pyrimidine synthesis. More specifically, HES4 directly represses transcription of SLC44A2 and SDS, thereby inhibiting mitochondrial choline oxidation and cytosolic serine deamination, respectively, which, in turn, ensures coenzyme Q reduction capacity for DHODH-mediated UMP synthesis and serine-derived dTMP production. Accordingly, inhibition of choline oxidation preserves mitochondrial serine catabolism and ETC-coupled redox balance. Furthermore, HES4 protein stability is enhanced under EGFR activation, and increased HES4 levels facilitate EGFR-driven tumor growth and predict poor prognosis of lung adenocarcinoma. These findings illustrate an unidentified mechanism, underlying pyrimidine biosynthesis in the intersection between serine and choline catabolism, and underscore the physiological importance of HES4 in tumor metabolism. The authors identify genes potentially involved in NAD(H) redox modulation and provide insight on major hit HES4, which uses its transcriptional repressive function to drive pyrimidine nucleotide biosynthesis and tumor growth.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


