HGSNAT 对溶酶体跨膜乙酰化的结构和机制
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
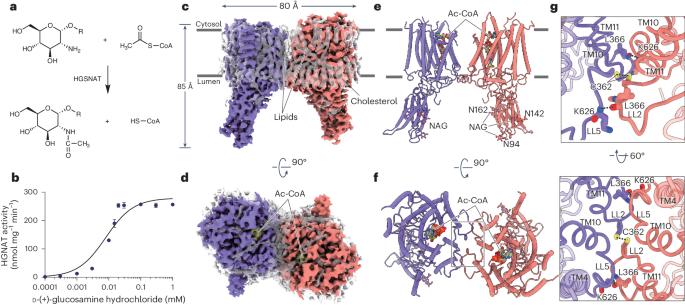
硫酸肝素(HS)的溶酶体跨膜乙酰化是由HS乙酰-CoA:α-氨基葡萄糖N-乙酰转移酶(HGSNAT)催化的。HGSNAT是HS降解过程中唯一的非水解酶,它将细胞质乙酰辅酶A(Ac-CoA)和溶酶体HS结合在一起进行N-乙酰转移酶反应的机制尚不清楚。在这里,我们展示了 HGSNAT 单独、与 Ac-CoA 复合物以及与乙酰化产物的低温电子显微镜结构。这些结构说明,来自细胞质一侧的 Ac-CoA 结合会导致二聚 HGSNAT 形成一个跨膜隧道。在该隧道中,催化组氨酸和天冬酰胺接近管腔,并促使乙酰基从 Ac-CoA 转移到 HS 的葡糖胺基团上。我们的研究揭示了一种跨膜乙酰化机制,它可能有助于推进针对溶酶体贮积疾病的治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Structure and mechanism of lysosome transmembrane acetylation by HGSNAT
Lysosomal transmembrane acetylation of heparan sulfates (HS) is catalyzed by HS acetyl-CoA:α-glucosaminide N-acetyltransferase (HGSNAT), whose dysfunction leads to lysosomal storage diseases. The mechanism by which HGSNAT, the sole non-hydrolase enzyme in HS degradation, brings cytosolic acetyl-coenzyme A (Ac-CoA) and lysosomal HS together for N-acyltransferase reactions remains unclear. Here, we present cryogenic-electron microscopy structures of HGSNAT alone, complexed with Ac-CoA and with acetylated products. These structures explain that Ac-CoA binding from the cytosolic side causes dimeric HGSNAT to form a transmembrane tunnel. Within this tunnel, catalytic histidine and asparagine approach the lumen and instigate the transfer of the acetyl group from Ac-CoA to the glucosamine group of HS. Our study unveils a transmembrane acetylation mechanism that may help advance therapeutic strategies targeting lysosomal storage diseases. This study reports the structure of lysosomal N-acetyltransferase HGSNAT providing insights into the mechanism of lysosomal transmembrane acetylation of heparan sulfate required for its catabolism.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


