通过锌(II)稳定脒基自由基促进脱氨基方法合成喹唑啉酮支架
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
在此,我们报告了一种完全以配体为中心的氧化还原控制有效方案,该方案利用一种台式稳定的 C2-酰胺化咪唑鎓盐支撑锌化合物,通过邻氨基酰胺/酯与腈的偶联,高产率地获得了多种药用相关支架--喹唑啉-4(3H)-酮。重要的是,详细的机理探究确定了通过氨基自由基形成的反应途径。此外,通过对所获产物进行后修饰以获得与治疗相关的复杂有机分子,展示了这些产物的合成用途。本文章由计算机程序翻译,如有差异,请以英文原文为准。
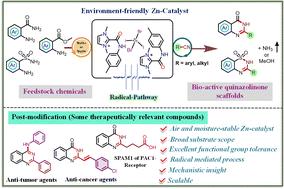
Synthesis of quinazolinone scaffolds via a zinc(ii)-stabilized amidyl radical-promoted deaminative approach†
Herein, we report a solely ligand centered redox controlled protocol, utilizing a bench stable zinc compound, for the efficient coupling of o-amino amides/esters with nitriles to afford diverse quinazolinone scaffolds and their synthetic utility was showcased via post-modification to access therapeutically relevant compounds. Importantly, mechanistic probes established the reaction pathway that proceeds via aminyl radical.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


