催化更快的动力
IF 38.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
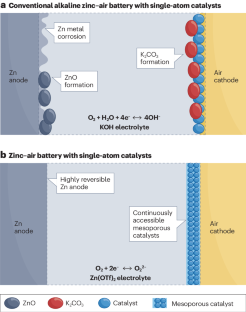
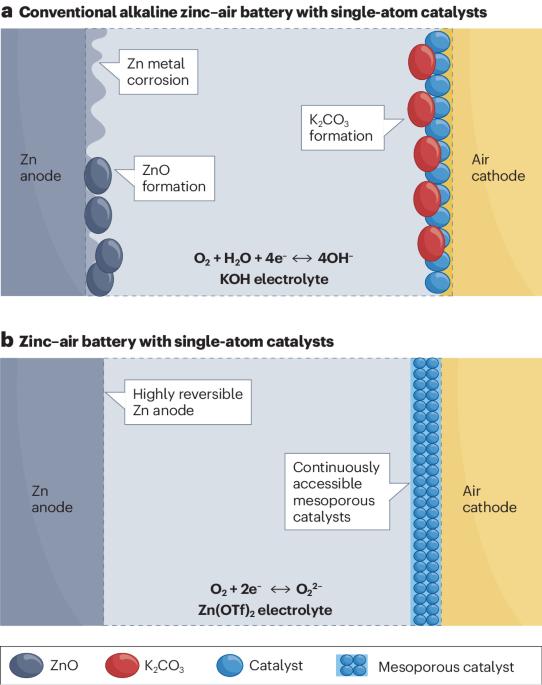
改进锌-空气电池具有挑战性,原因在于动力学和有限的电化学可逆性,这部分归因于缓慢的四电子氧化还原化学。现在,双电子氧化还原化学和催化剂取得了长足进步,使锌-空气电池达到了前所未有的稳定性,能效高达 61%。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Catalytically faster power
Improving zinc–air batteries is challenging due to kinetics and limited electrochemical reversibility, partly attributed to sluggish four-electron redox chemistry. Now, substantial strides are noted with two-electron redox chemistry and catalysts, resulting in unprecedentedly stable zinc–air batteries with 61% energy efficiencies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature reviews. Chemistry
Chemical Engineering-General Chemical Engineering
CiteScore
52.80
自引率
0.80%
发文量
88
期刊介绍:
Nature Reviews Chemistry is an online-only journal that publishes Reviews, Perspectives, and Comments on various disciplines within chemistry. The Reviews aim to offer balanced and objective analyses of selected topics, providing clear descriptions of relevant scientific literature. The content is designed to be accessible to recent graduates in any chemistry-related discipline while also offering insights for principal investigators and industry-based research scientists. Additionally, Reviews should provide the authors' perspectives on future directions and opinions regarding the major challenges faced by researchers in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


