常驻和浸润免疫细胞通过 IL-18R/IFN-γ/ROS 轴在小鼠体内防御长须鲸军团菌的过程中发挥相反的作用。
IF 7.6
2区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
长须鲸军团菌是经常导致死亡的军团菌肺炎的致病菌,人们对这种病菌的免疫反应知之甚少。在这里,我们研究了组织驻留的肺泡巨噬细胞(AM)和浸润吞噬细胞在感染这种病原体时的特殊作用。AM是感染一天后内化细菌的主要细胞类型。感染 3 天和 5 天后,AM 的数量大大减少,而中性粒细胞和随后的单核细胞衍生细胞(MC)则大量涌入肺组织。与中性粒细胞和单核细胞相比,AM携带的存活长须杆菌数量更多,这与长须杆菌将细菌复制所需的细菌效应蛋白转运到AM细胞膜的能力更强有关。细胞消融实验表明,AM 能促进感染,而中性粒细胞和 MC 则是有效清除细菌的必要条件。IL-18对IL-18R+ NK细胞和T细胞产生IFN-γ非常重要,这反过来又刺激了中性粒细胞中ROS介导的杀菌活性,从而限制了L.longbeachae的感染。纤毛支气管上皮细胞也表达 IL-18R,但在 IL-18 介导的 L.longbeachae 清除过程中并未发挥作用。我们的研究结果发现,在L.longbeachae感染过程中,组织驻留免疫细胞和浸润免疫细胞的先天功能是相反的,可以通过调节这些功能来改善保护性反应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
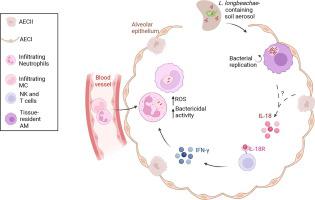
Opposing roles of resident and infiltrating immune cells in the defense against Legionella longbeachae via IL-18R/IFN-γ/ROS axis in mice
The immune response against Legionella longbeachae, a causative agent of the often-fatal Legionnaires’ pneumonia, is poorly understood. Here, we investigated the specific roles of tissue-resident alveolar macrophages (AMs) and infiltrating phagocytes during infection with this pathogen. AMs were the predominant cell type that internalized bacteria 1 day after infection. A total of 3 and 5 days after infection, AM numbers were greatly reduced, whereas there was an influx of neutrophils and, later, monocyte-derived cells (MCs) into lung tissue. AMs carried greater numbers of viable L. longbeachae than neutrophils and MCs, which correlated with a higher capacity of L. longbeachae to translocate bacterial effector proteins required for bacterial replication into the AM cytosol. Cell ablation experiments demonstrated that AM promoted infection, whereas neutrophils and MC were required for efficient bacterial clearance. Interleukin (IL)-18 was important for interferon-γ production by IL-18R+ natural killer cells and T cells, which, in turn, stimulated reactive oxygen species–mediated bactericidal activity in neutrophils, resulting in the restriction of L. longbeachae infection. Ciliated bronchiolar epithelial cells also expressed IL-18R but did not play a role in IL-18–mediated L. longbeachae clearance. Our results have identified opposing innate functions of tissue-resident and infiltrating immune cells during L. longbeachae infection that may be manipulated to improve protective responses.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Mucosal Immunology
医学-免疫学
CiteScore
16.60
自引率
3.80%
发文量
100
审稿时长
12 days
期刊介绍:
Mucosal Immunology, the official publication of the Society of Mucosal Immunology (SMI), serves as a forum for both basic and clinical scientists to discuss immunity and inflammation involving mucosal tissues. It covers gastrointestinal, pulmonary, nasopharyngeal, oral, ocular, and genitourinary immunology through original research articles, scholarly reviews, commentaries, editorials, and letters. The journal gives equal consideration to basic, translational, and clinical studies and also serves as a primary communication channel for the SMI governing board and its members, featuring society news, meeting announcements, policy discussions, and job/training opportunities advertisements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


