上皮组织中活跃的孔洞形成
IF 17.6
1区 物理与天体物理
Q1 PHYSICS, MULTIDISCIPLINARY
引用次数: 0
摘要
上皮组织中孔的形成对发育至关重要,但它也可能与上皮屏障功能障碍和癌症进展有关。在这里,我们展示了源自人类胚胎干细胞的上皮单层组织中活跃的细胞收缩可自发地启动一个形态转变级联,包括孔核化、凝聚和网络形成。累积的组织级拉伸应力推动孔洞从各向同性的圆形扩展到细胞间连接的局部断裂。随后是快速的裂纹扩展,这种扩展后来被自组织的细胞上肌动蛋白环所抑制,并伴随着裂纹钝化和断裂到圆形的转变。在孔凝聚过程中,我们发现了一种断裂-滑动机制,它能使多细胞桥逐层断裂,但不会导致细胞过度变形。我们的多尺度理论捕捉到了这些实验观察结果,并预测基质刚度感应和细胞粘附与细胞收缩竞争,从而介导了形态动力学。这些研究结果表明,活体组织可以协调分子、细胞和组织尺度的力学,以驱动拓扑变化,同时降低对细胞造成机械损伤的风险。本文章由计算机程序翻译,如有差异,请以英文原文为准。
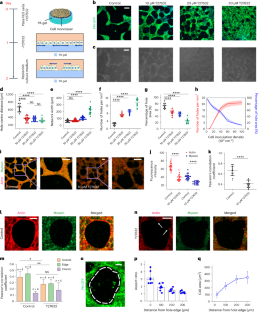
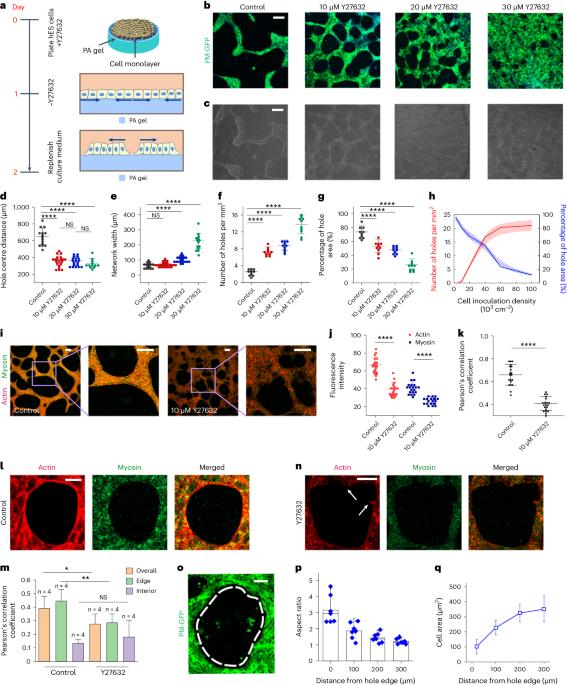
Active hole formation in epithelioid tissues
The formation of holes in epithelial tissue is essential for development, but it can also be associated with epithelial barrier dysfunction and cancer progression. Here we show that active cell contraction in epithelioid monolayer tissues derived from human embryonic stem cells can spontaneously launch a morphological transition cascade consisting of hole nucleation, coalescence and network formation. Accumulated tissue-level tensile stresses drive hole expansion from isotropic round expansion to local fracture of intercellular junctions. This is followed by fast crack propagation, which is later suppressed by the self-organized supracellular actomyosin ring and accompanied by crack blunting and a fracture-to-rounding transition. During hole coalescence, we find a fracture–slip mechanism that enables layer-by-layer breaking of the multicellular bridge but without inducing excessive cell deformation. Our multiscale theory captures these experimental observations and predicts that substrate rigidity sensing and adhesion of cells compete with cellular contraction to mediate the morphological dynamics. These findings suggest that living tissues may coordinate the mechanics across molecular, cellular and tissue scales to drive topological changes while reducing the risk of mechanical damage to cells. Active cell contraction drives hole nucleation, fracture and crack propagation in a tissue monolayer through a process reminiscent of dewetting thin films.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Physics
物理-物理:综合
CiteScore
30.40
自引率
2.00%
发文量
349
审稿时长
4-8 weeks
期刊介绍:
Nature Physics is dedicated to publishing top-tier original research in physics with a fair and rigorous review process. It provides high visibility and access to a broad readership, maintaining high standards in copy editing and production, ensuring rapid publication, and maintaining independence from academic societies and other vested interests.
The journal presents two main research paper formats: Letters and Articles. Alongside primary research, Nature Physics serves as a central source for valuable information within the physics community through Review Articles, News & Views, Research Highlights covering crucial developments across the physics literature, Commentaries, Book Reviews, and Correspondence.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


