首次全合成阿斯佩里内酯 B。修订阿斯佩里内酯 B 和 C 的绝对立体化学。
IF 2.9
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
首次完成了阿斯佩里内酯 B (I) 的全合成和绝对构型分配。此外,还对阿斯佩里内酯 C 的绝对立体化学进行了修订。此外,还首次合成了阿斯佩里内酯 B 的对映体(ent-I),以及阿斯佩里内酯 B (8) 和 C (9) 的 C-7 外比体。本文章由计算机程序翻译,如有差异,请以英文原文为准。
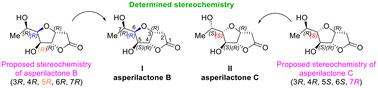
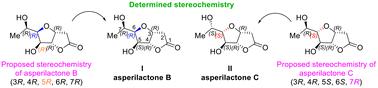
First total synthesis of asperilactone B. Revision of absolute stereochemistry of asperilactones B and C†
The first total synthesis and absolute configuration assignment of asperilactone B (I) have been accomplished. Additionally, a revision of the absolute stereochemistry of asperilactone C has been done. The first total synthesis of the opposite enantiomer of asperilactone B (ent-I) has also been achieved, as well as that of C-7 epimers of both asperilactones B (8) and C (9).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
The international home of synthetic, physical and biomolecular organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


