链霉菌中含有不寻常的 N-甲基-3-甲基亚磺酰基丙氨酸残基的喹霉素
IF 2.1
4区 医学
Q3 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
摘要
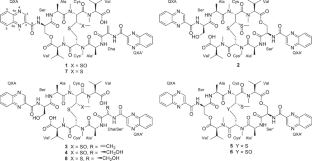
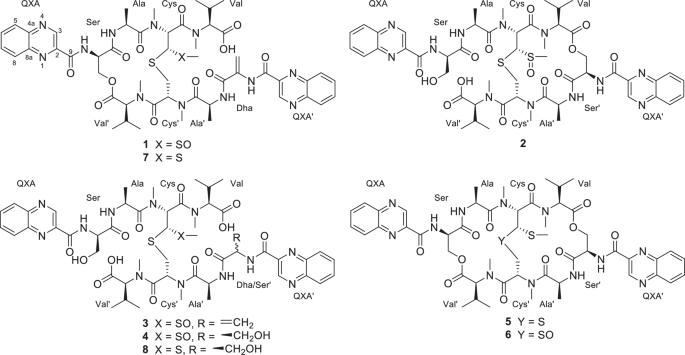
从源于土壤的链霉菌 CPCC205575 的培养物中分离出四种新的棘霉素同系物--喹霉素 M-P (1-4)。通过对 NMR 和 HRESIMS/MS 数据进行综合分析,确定了它们的平面结构。通过先进的 Marfey 方法并结合生物合成基因分析,阐明了化合物的绝对构型。化合物 1-4 代表了喹诺霉素类天然产物的第一个实例,其 N,S-二甲基半胱氨酸残基上的硫原子被氧化为一个亚砜基团,形成了不寻常的 N-甲基-3-甲基亚砜基-丙氨酸残基。生物测定结果表明,Cys 或 Cys'残基上的硫原子被氧化后,细胞毒性和抗菌活性会急剧下降。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Quinomycins with an unusual N-methyl-3-methylsulfinyl-alanine residue from a Streptomyces sp
Four new echinomycin congeners, quinomycins M−P (1−4) were isolated from the cultures of the soil-derived Streptomyces sp. CPCC205575. The planar structures were determined by comprehensive analyses of NMR and HRESIMS/MS data. The absolute configurations were elucidated by the advanced Marfey’s method combined with biosynthetic gene analysis. Compounds 1−4 represent the first examples of quinomycin-type natural products with the sulfur atom at the N,S-dimethylcysteine residue oxidized as a sulfoxide group forming the unusual N-methyl-3-methylsulfinyl-alanine residue. Bioassay results revealed that the oxidation of the sulfur atom at the Cys or Cys′ residues led to dramatic decrease of cytotoxicity and antimicrobial activity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Antibiotics
医学-免疫学
CiteScore
6.60
自引率
3.00%
发文量
87
审稿时长
1 months
期刊介绍:
The Journal of Antibiotics seeks to promote research on antibiotics and related types of biologically active substances and publishes Articles, Review Articles, Brief Communication, Correspondence and other specially commissioned reports. The Journal of Antibiotics accepts papers on biochemical, chemical, microbiological and pharmacological studies. However, studies regarding human therapy do not fall under the journal’s scope. Contributions regarding recently discovered antibiotics and biologically active microbial products are particularly encouraged. Topics of particular interest within the journal''s scope include, but are not limited to, those listed below:
Discovery of new antibiotics and related types of biologically active substances
Production, isolation, characterization, structural elucidation, chemical synthesis and derivatization, biological activities, mechanisms of action, and structure-activity relationships of antibiotics and related types of biologically active substances
Biosynthesis, bioconversion, taxonomy and genetic studies on producing microorganisms, as well as improvement of production of antibiotics and related types of biologically active substances
Novel physical, chemical, biochemical, microbiological or pharmacological methods for detection, assay, determination, structural elucidation and evaluation of antibiotics and related types of biologically active substances
Newly found properties, mechanisms of action and resistance-development of antibiotics and related types of biologically active substances.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


