参与者对超低剂量联合疗法治疗高血压的看法:QUARTET 试验的混合方法研究。
IF 2.7
4区 医学
Q2 PERIPHERAL VASCULAR DISEASE
引用次数: 0
摘要
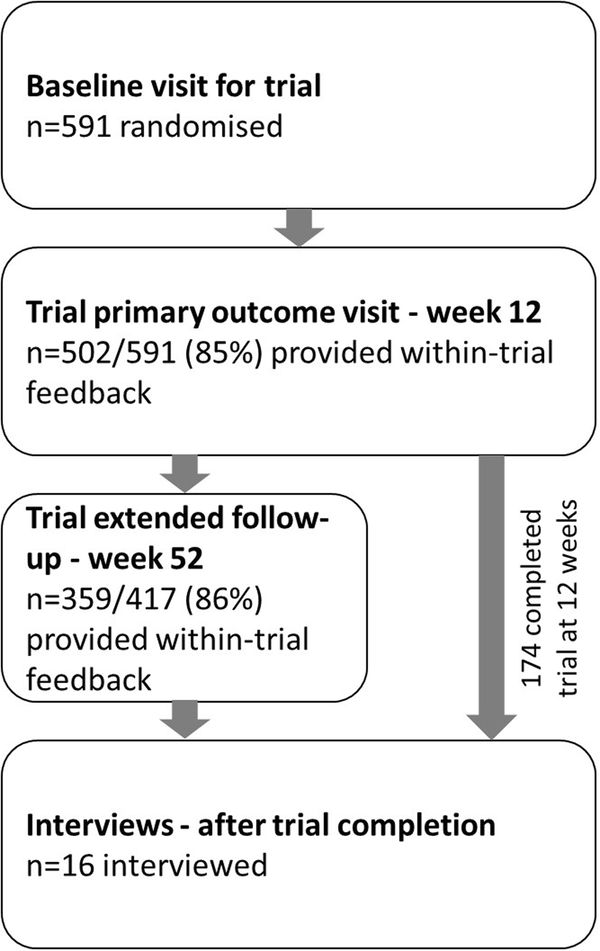
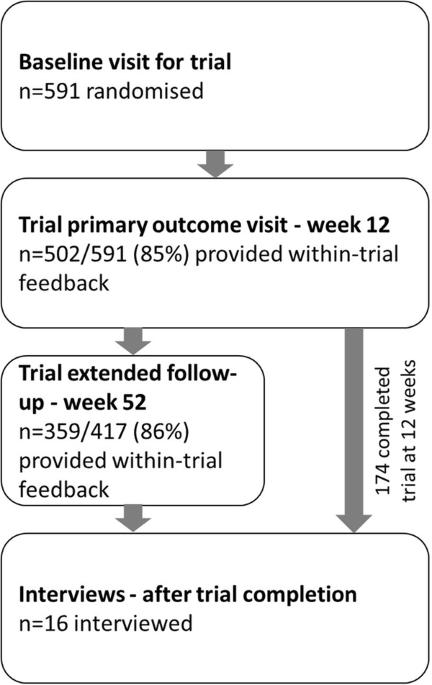
与单药治疗相比,含有四种四分之一剂量药物的高血压单药联合疗法可改善血压控制,但患者对四药联合疗法作为一种治疗策略的接受程度尚无定论。我们从四联超低剂量高血压治疗(QUARTET)试验中收集了试验反馈并对参与者进行了访谈,以了解患者对这种干预措施的态度。我们询问了所有试验参与者使用四联药物的难易程度和偏好,并让他们有机会在 12 周(试验主要终点)和 52 周延长随访时对试验提出进一步意见。另外,我们在半结构化电话访谈中使用了目的性抽样和配额抽样,并使用归纳式主题分析方法对由此产生的逐字记录进行了分析。在每次连续访谈后,我们都会对主题进行重新评估,在疑似数据饱和时,我们会再进行一次访谈以进行确认。随访 12 周时,591 名参与者中有 502 人(85%)回答了可接受性问题,417 名参与者中有 359 人(86%)在第 52 周时回答了可接受性问题。大多数人表示试验胶囊容易服用或非常容易服用。在 2020 年 8 月 5 日至 2020 年 11 月 19 日期间,对 8 个地点的 16 名参与者进行了访谈。所有参与者都表示体验良好,喜欢每天早上服药一次,并认为常规服药有助于坚持服药。参与者重视个人对坚持服药的责任以及全科医生对血压管理的参与。大多数人表示,胶囊的大小并不妨碍坚持服药,但希望能有更小的胶囊。据参与者描述,他们更倾向于尽量减少药物的数量和剂量、减小胶囊大小以及每天早上服药一次。这些研究结果表明,人们更倾向于采用单药联合疗法来降低血压。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Participants’ views of ultra-low dose combination therapy for high blood pressure: a mixed-methods study from the QUARTET trial
Single-pill combination therapy containing four quarter-dose medications for high blood pressure improves BP control compared to monotherapy, however patient-reported acceptance of the quadpill as a treatment strategy remains undescribed. We collected within-trial feedback and interviewed participants from the quadruple ultra-low-dose treatment for hypertension (QUARTET) trial to characterise patient attitudes to this intervention. All trial participants were asked about ease and preference for the quadpill and provided an opportunity to give further comments on the trial at 12 weeks (trial primary endpoint) and 52 weeks extended follow-up. Separately, we used purposive and quota sampling for the semi-structured telephone interviews, with the resultant verbatim transcripts analysed using an inductive thematic analysis approach. Themes were re-evaluated after each successive interview, and at suspected data saturation, an additional interview conducted for confirmation. At 12 weeks follow-up, 502 of 591 (85%) participants responded to acceptability questions, and 359 of 417 (86%) responded at week 52. Most reported the trial capsule easy or very easy to take. From eight sites, 16 participants were interviewed between 5 August 2020 and 19 November 2020. All described a positive experience, preferred once-daily morning dosing and found routine facilitated adherence. Participants valued individual responsibility for adherence, and involvement of the general practitioner in blood-pressure management. Most reported capsule size did not deter adherence but desired a smaller capsule. Participants described a preference for minimising number and dosage of medications, reduced capsule size, and once-daily morning dosing. These findings suggest a preference for single-pill combination therapy for blood pressure lowering.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Human Hypertension
医学-外周血管病
CiteScore
5.20
自引率
3.70%
发文量
126
审稿时长
6-12 weeks
期刊介绍:
Journal of Human Hypertension is published monthly and is of interest to health care professionals who deal with hypertension (specialists, internists, primary care physicians) and public health workers. We believe that our patients benefit from robust scientific data that are based on well conducted clinical trials. We also believe that basic sciences are the foundations on which we build our knowledge of clinical conditions and their management. Towards this end, although we are primarily a clinical based journal, we also welcome suitable basic sciences studies that promote our understanding of human hypertension.
The journal aims to perform the dual role of increasing knowledge in the field of high blood pressure as well as improving the standard of care of patients. The editors will consider for publication all suitable papers dealing directly or indirectly with clinical aspects of hypertension, including but not limited to epidemiology, pathophysiology, therapeutics and basic sciences involving human subjects or tissues. We also consider papers from all specialties such as ophthalmology, cardiology, nephrology, obstetrics and stroke medicine that deal with the various aspects of hypertension and its complications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


