相分离能力强的 FBL 可促进急性髓性白血病的早期前 RNA 处理和翻译
IF 19.1
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
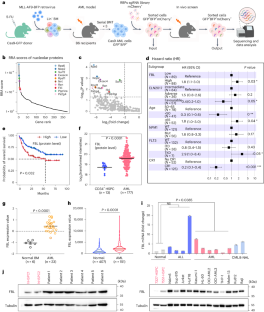
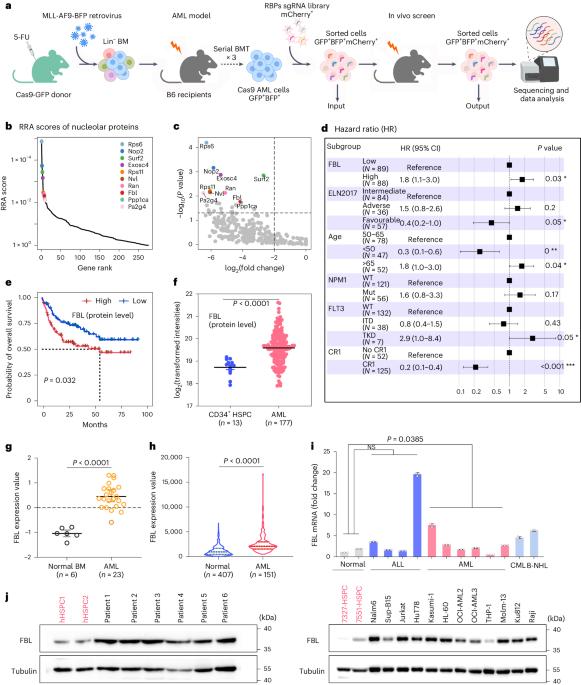
RNA 结合蛋白(RBPs)在急性髓性白血病(AML)这一致命疾病中起着关键作用。虽然在急性髓性白血病中发现了特异性相分离功能的 RBPs,但它们的凝集物形成对急性髓性白血病白血病生成的影响以及抑制相分离的治疗潜力尚未得到充分探索。在我们的体内 CRISPR RBP 筛选中,纤丝蛋白(FBL)成为调节急性髓细胞性白血病细胞存活的关键核仁蛋白,它主要通过相分离结构域而不是甲基转移酶或乙酰化结构域来调节细胞存活。这些具有特殊功能的相分离结构域能协调地驱动核小体的形成和pre-rRNA的早期处理(包括外流、裂解和甲基化),最终促进MYC等致癌基因的翻译。CGX-635 以 FBL 的相分离能力为靶点,可消除急性髓细胞白血病细胞,这表明 CGX-635 还有一种作用机制可补充其既有的治疗效果。我们强调了 PS 调节关键蛋白作为急性髓细胞性白血病可能治疗策略的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Phase separation-competent FBL promotes early pre-rRNA processing and translation in acute myeloid leukaemia
RNA-binding proteins (RBPs) are pivotal in acute myeloid leukaemia (AML), a lethal disease. Although specific phase separation-competent RBPs are recognized in AML, the effect of their condensate formation on AML leukaemogenesis, and the therapeutic potential of inhibition of phase separation are underexplored. In our in vivo CRISPR RBP screen, fibrillarin (FBL) emerges as a crucial nucleolar protein that regulates AML cell survival, primarily through its phase separation domains rather than methyltransferase or acetylation domains. These phase separation domains, with specific features, coordinately drive nucleoli formation and early processing of pre-rRNA (including efflux, cleavage and methylation), eventually enhancing the translation of oncogenes such as MYC. Targeting the phase separation capability of FBL with CGX-635 leads to elimination of AML cells, suggesting an additional mechanism of action for CGX-635 that complements its established therapeutic effects. We highlight the potential of PS modulation of critical proteins as a possible therapeutic strategy for AML. Yang et al. report that the nucleolar protein fibrillarin (FBL) affects acute myeloid leukaemia (AML) cell function through biomolecular condensation-dependent regulation of early pre-rRNA processing and translation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


