荚膜细胞靶向疗法--进展与未来方向
IF 28.6
1区 医学
Q1 UROLOGY & NEPHROLOGY
引用次数: 0
摘要
荚膜细胞是原发性和继发性蛋白尿肾脏疾病的主要损伤靶细胞,占全球肾衰竭病例的 90%。重要的实验和临床研究已经证实,在进行性肾小球疾病中,荚膜细胞耗竭与蛋白尿程度之间存在因果关系。然而,肾小球疾病的治疗方法并没有取得实质性进展,荚膜细胞病的标准治疗方法依赖于重新定位的免疫抑制药物。过去二十年来,人们对荚膜细胞损伤机理基础的认识有了显著提高,基于精准治疗方法的前景也日益光明。目前已鉴定出数十种在临床荚膜病发病机制中发挥作用的致病基因,以及一些假定的肾小球通透性因子。这些成就,加上荚膜细胞损伤新靶点的确定、利用祖细胞群的内源性荚膜细胞再生潜力的潜在方法的开发、正在进行的荚膜细胞特异性药剂临床试验以及荚膜细胞定向给药系统的开发,使得未来的肾小球疾病治疗前景乐观。本文章由计算机程序翻译,如有差异,请以英文原文为准。
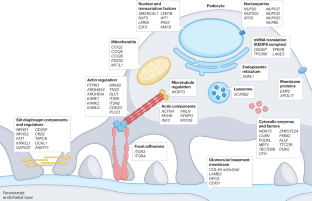
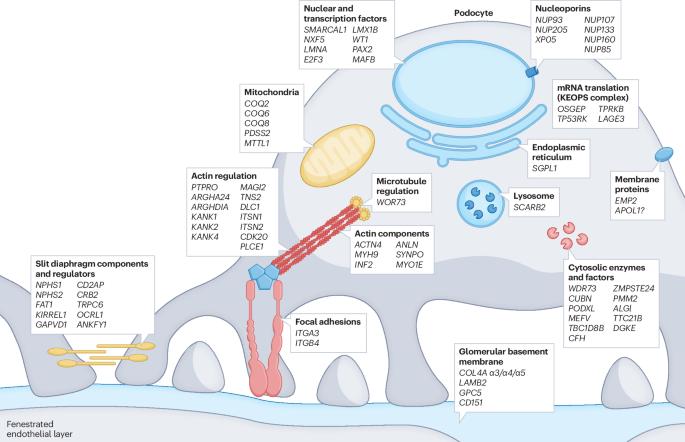
Podocyte-targeted therapies — progress and future directions
Podocytes are the key target cells for injury across the spectrum of primary and secondary proteinuric kidney disorders, which account for up to 90% of cases of kidney failure worldwide. Seminal experimental and clinical studies have established a causative link between podocyte depletion and the magnitude of proteinuria in progressive glomerular disease. However, no substantial advances have been made in glomerular disease therapies, and the standard of care for podocytopathies relies on repurposed immunosuppressive drugs. The past two decades have seen a remarkable expansion in understanding of the mechanistic basis of podocyte injury, with prospects increasing for precision-based treatment approaches. Dozens of disease-causing genes with roles in the pathogenesis of clinical podocytopathies have been identified, as well as a number of putative glomerular permeability factors. These achievements, together with the identification of novel targets of podocyte injury, the development of potential approaches to harness the endogenous podocyte regenerative potential of progenitor cell populations, ongoing clinical trials of podocyte-specific pharmacological agents and the development of podocyte-directed drug delivery systems, contribute to an optimistic outlook for the future of glomerular disease therapy. In this Review, the authors summarize the mechanistic rationale for current treatments for podocytopathies and for novel podocyte-targeted therapies. They also discuss potential approaches to regenerate podocytes and to develop podocyte-specific drug delivery systems.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Reviews Nephrology
医学-泌尿学与肾脏学
CiteScore
39.00
自引率
1.20%
发文量
127
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Nephrology aims to be the premier source of reviews and commentaries for the scientific communities it serves.
It strives to publish authoritative, accessible articles.
Articles are enhanced with clearly understandable figures, tables, and other display items.
Nature Reviews Nephrology publishes Research Highlights, News & Views, Comments, Reviews, Perspectives, and Consensus Statements.
The content is relevant to nephrologists and basic science researchers.
The broad scope of the journal ensures that the work reaches the widest possible audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


