睡眠期间海马表象的再调整
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
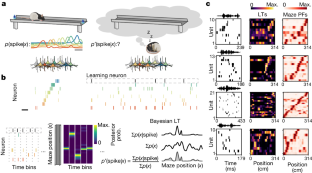
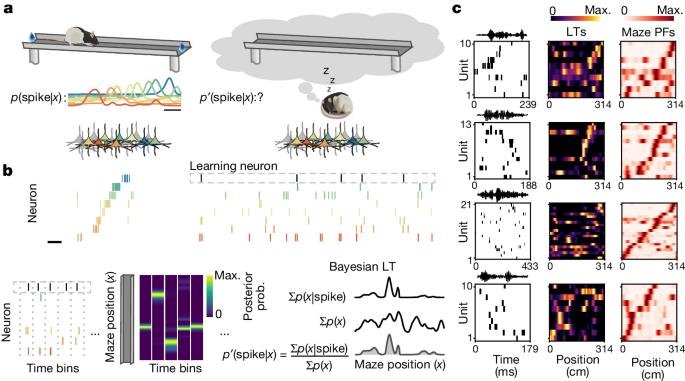
作为空间记忆基础的海马表征在形成后会不断完善1。在这里,为了追踪神经元在离线状态下的动态空间调谐,我们使用了一种新的贝叶斯学习方法,该方法基于自由移动大鼠的集合记录中的尖峰触发平均解码位置。通过测量这些调谐,我们发现海马锐波涟漪中的空间表征在睡眠期间稳定数小时,并且与迷宫探索期间最初观察到的位置场高度一致。这些表征是由多种因素组合而成的,其中包括迷宫暴露前的预配置结构,以及在迷宫中出现的θ-振荡和清醒锐波波纹表征,揭示了这些事件在形成集合时的贡献。令人震惊的是,睡眠时的波纹表征可以预测神经元在再次暴露于迷宫时的未来位置场,即使这些位置场偏离了之前的位置偏好。相比之下,我们在迷宫暴露前的睡眠和休息期间以及睡眠后期观察到的调谐与迷宫位置场的一致性较差。总之,新的解码方法使我们能够推断和描述离线期场所场的稳定性和重新调整,揭示了新探索后表征的快速出现以及睡眠在海马表征动态中的作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Retuning of hippocampal representations during sleep
Hippocampal representations that underlie spatial memory undergo continuous refinement following formation1. Here, to track the spatial tuning of neurons dynamically during offline states, we used a new Bayesian learning approach based on the spike-triggered average decoded position in ensemble recordings from freely moving rats. Measuring these tunings, we found spatial representations within hippocampal sharp-wave ripples that were stable for hours during sleep and were strongly aligned with place fields initially observed during maze exploration. These representations were explained by a combination of factors that included preconfigured structure before maze exposure and representations that emerged during θ-oscillations and awake sharp-wave ripples while on the maze, revealing the contribution of these events in forming ensembles. Strikingly, the ripple representations during sleep predicted the future place fields of neurons during re-exposure to the maze, even when those fields deviated from previous place preferences. By contrast, we observed tunings with poor alignment to maze place fields during sleep and rest before maze exposure and in the later stages of sleep. In sum, the new decoding approach allowed us to infer and characterize the stability and retuning of place fields during offline periods, revealing the rapid emergence of representations following new exploration and the role of sleep in the representational dynamics of the hippocampus. Using a Bayesian learning approach, a study tracks the spatial representations by individual hippocampal cells over time in freely moving rats, and provides insights into how ensemble patterns form and reconfigure during sleep.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


