DNA ADP-核糖基化失调会损害端粒复制
IF 10.1
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
人们认识到 DNA 可以被 ADP 核糖基化,这为了解 ADP 核糖基化如何促进基因组稳定性、表观遗传学和免疫提供了一个意想不到的调控层面。然而,DNA ADP-核糖基化(DNA-ADPr)是否会促进基因组的稳定性以及它是如何被调控的仍是未知数。在这里,我们发现端粒会在 PARP1 的催化下发生 DNA-ADPr,并被 TARG1 清除。从机理上讲,我们发现DNA-ADPr与滞后端粒DNA链的合成相关联,在未连接的冈崎片段和3′单链端粒悬空处形成单链DNA。由于缺乏TARG1,DNA连接的ADPr持续存在,最终导致端粒缩短。此外,我们还利用细菌 DNA ADP-核糖基转移酶毒素直接修饰端粒的 DNA,证明未水解的 DNA 链接 ADP-ribose 会损害端粒复制和端粒完整性。因此,我们的研究通过确定端粒是 PARP1 和 TARG1 调节的 DNA-ADPr 的染色体靶标(其失调会损害端粒的复制和完整性),强调并确定了控制 DNA-ADPr 的周转对于持续的基因组稳定性至关重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
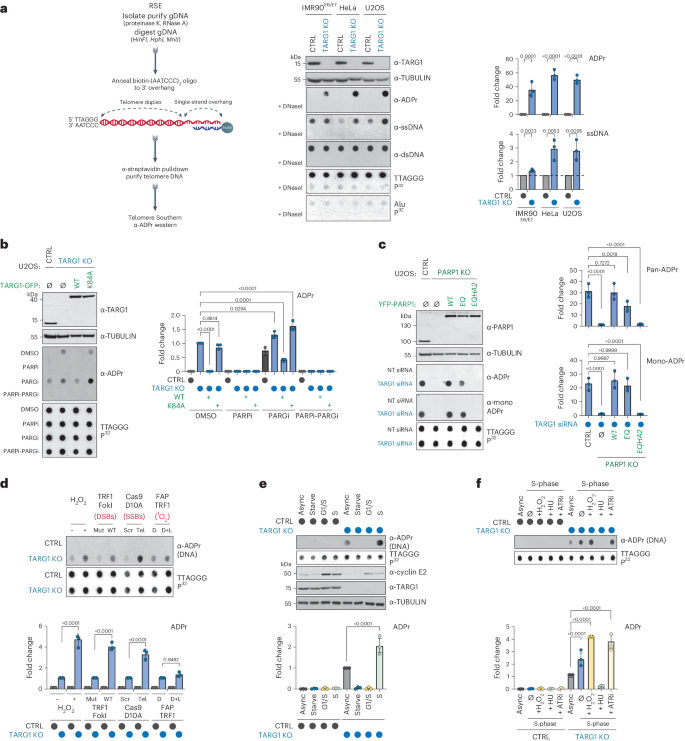
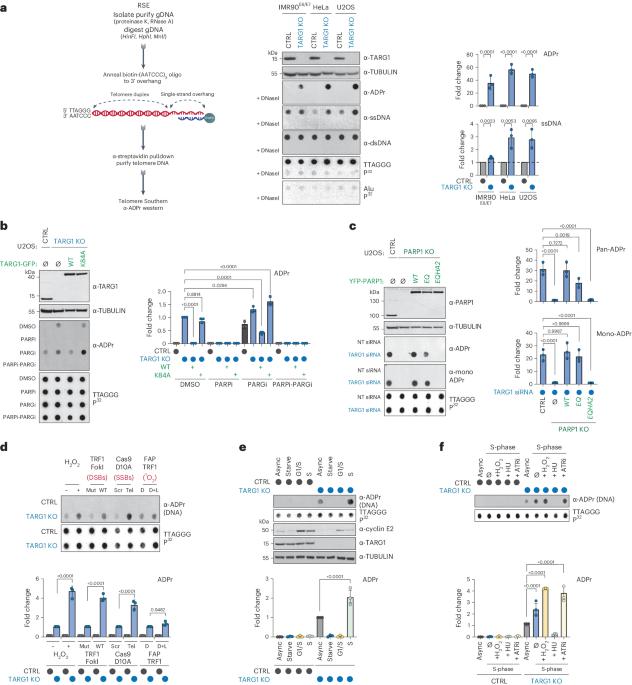
Deregulated DNA ADP-ribosylation impairs telomere replication
The recognition that DNA can be ADP ribosylated provides an unexpected regulatory level of how ADP-ribosylation contributes to genome stability, epigenetics and immunity. Yet, it remains unknown whether DNA ADP-ribosylation (DNA-ADPr) promotes genome stability and how it is regulated. Here, we show that telomeres are subject to DNA-ADPr catalyzed by PARP1 and removed by TARG1. Mechanistically, we show that DNA-ADPr is coupled to lagging telomere DNA strand synthesis, forming at single-stranded DNA present at unligated Okazaki fragments and on the 3′ single-stranded telomere overhang. Persistent DNA-linked ADPr, due to TARG1 deficiency, eventually leads to telomere shortening. Furthermore, using the bacterial DNA ADP-ribosyl-transferase toxin to modify DNA at telomeres directly, we demonstrate that unhydrolyzed DNA-linked ADP-ribose compromises telomere replication and telomere integrity. Thus, by identifying telomeres as chromosomal targets of PARP1 and TARG1-regulated DNA-ADPr, whose deregulation compromises telomere replication and integrity, our study highlights and establishes the critical importance of controlling DNA-ADPr turnover for sustained genome stability. Telomeres are endogenous cellular targets of DNA ADP-ribosylation (DNA-ADPr). TARG1-regulated DNA-ADPr is coupled to lagging telomere DNA strand synthesis, and persistent DNA-ADPr, due to TARG1 deficiency, leads to telomere shortening and fragility.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


