ADP-核糖基化端粒 DNA 的风险业务
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
ADP-核糖基化调节着许多参与DNA损伤反应和修复的蛋白质的活性。一项新的研究表明,端粒DNA可被PARP1进行ADP-核糖化,而TARG1及时清除ADP-核糖对保持端粒完整性至关重要,这揭示了DNA-ADP-核糖化是端粒稳定性的一个新角色。本文章由计算机程序翻译,如有差异,请以英文原文为准。
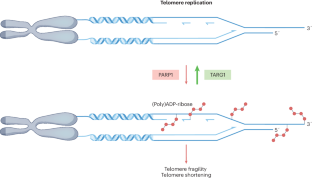
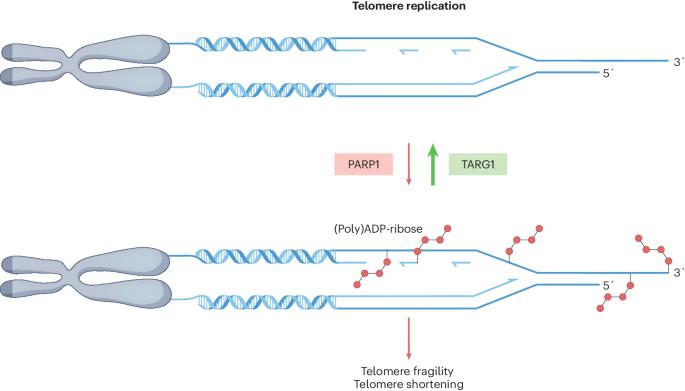
The risky business of ADP-ribosylating telomeric DNA
ADP-ribosylation regulates the activity of numerous proteins involved in the DNA damage response and repair. A new study shows that telomeric DNA can be ADP-ribosylated by PARP1, and prompt removal of the ADP-ribose by TARG1 is essential to preserve telomere integrity, unveiling DNA–ADP-ribosylation as a novel player in telomere stability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


