转移性定植的细胞内在因素和微环境决定因素
IF 17.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
癌症转移是一个复杂的生物学过程,仍然是肿瘤临床上的一大挑战,几乎占恶性肿瘤相关死亡率的全部。要建立转移性生长,癌细胞必须从原发肿瘤扩散,在陌生的组织微环境中存活,重新激活增殖程序,并逃避先天性和适应性免疫监视。整个过程的效率极低,时间跨度长,只有极少数癌细胞能完成所有必要步骤。在此,我们回顾了癌细胞内在机制和微环境相互作用促成转移定植的过程。我们特别强调了最近关于已经扩散的肿瘤细胞行为的研究,因为要在治疗转移性疾病方面取得有意义的进展,显然需要更好地了解产生转移的细胞,这些细胞通常在最初诊断时就已经扩散。本文章由计算机程序翻译,如有差异,请以英文原文为准。
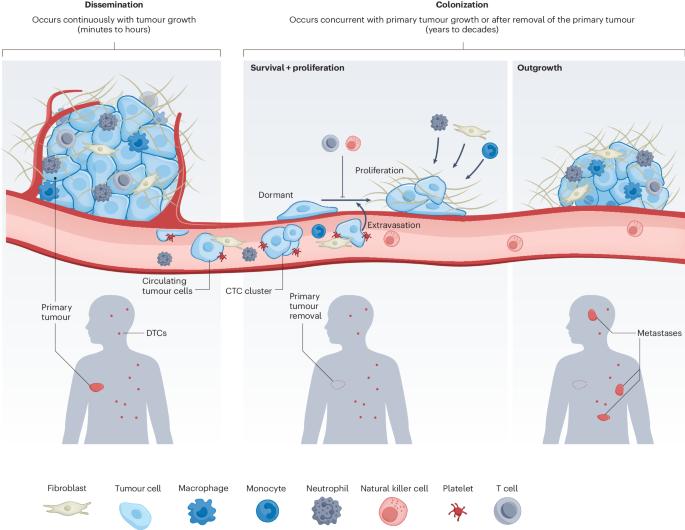
Cell-intrinsic and microenvironmental determinants of metastatic colonization
Cancer metastasis is a biologically complex process that remains a major challenge in the oncology clinic, accounting for nearly all of the mortality associated with malignant neoplasms. To establish metastatic growths, carcinoma cells must disseminate from the primary tumour, survive in unfamiliar tissue microenvironments, re-activate programs of proliferation, and escape innate and adaptive immunosurveillance. The entire process is extremely inefficient and can occur over protracted timescales, yielding only a vanishingly small number of carcinoma cells that are able to complete all of the required steps. Here we review both the cancer-cell-intrinsic mechanisms and microenvironmental interactions that enable metastatic colonization. In particular, we highlight recent work on the behaviour of already-disseminated tumour cells, since meaningful progress in treating metastatic disease will clearly require a better understanding of the cells that spawn metastases, which generally have disseminated by the time of initial diagnosis. Metastatic colonization involves cancer-cell-intrinsic mechanisms and microenvironmental interactions, and a better understanding of the factors that influence the final, post-extravasation phases is crucial for therapeutically targeting metatstasis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


