在酸性电解质中利用铠甲状铁纳米颗粒/多孔掺氮碳实现高效的二氧化碳电化学还原
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
二氧化碳的电化学还原在实现高选择性和稳定性方面一直存在挑战,尤其是在酸性电解质中。在此,我们采用无溶剂机械化学方法,在掺氮碳(Fe@NC)内成功设计出一种高效的铠甲状催化剂。多孔掺氮碳壳可作为铁纳米颗粒的有效保护层,促进二氧化碳向一氧化碳的转化,在酸性电解质中的FECO高达99.0%,令人印象深刻。因此,铠装的 Fe@NC 在 14 小时的电解过程中一直保持着催化活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
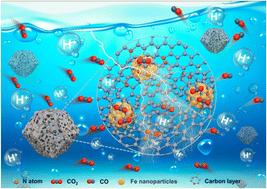
Efficient electrochemical CO2 reduction in acidic electrolytes using armor-like iron nanoparticles/porous nitrogen-doped carbon†
Electrochemical reduction of CO2 presents a persistent challenge in achieving high selectivity and stability, particularly in acidic electrolytes. Here, we successfully engineer an efficient armor-like catalyst, comprising Fe nanoparticles within nitrogen-doped carbon (Fe@NC) based on a solvent-free mechanochemistry method followed by pyrolysis. Porous nitrogen-doped carbon shells served as an effective protective layer for the Fe nanoparticles, facilitating the conversion of CO2 to CO with an impressive FECO of 99.0% in acidic electrolytes. As a result, the armored Fe@NC sustained its catalytic activity throughout 14 hours electrolysis period.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


