基于远红弓形虫核素-3 的荧光基因编码电压指示器的合理设计:从阐明弓形虫的荧光机制到新型红移变体
IF 3.7
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
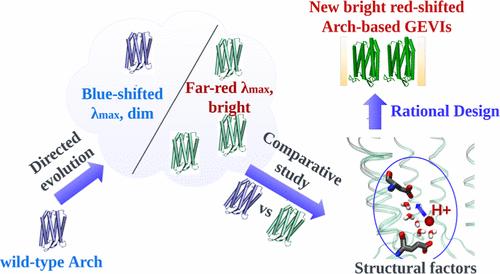
基因编码电压指示剂(GEVIs)作为可视化细胞膜电位变化的分子工具得到了广泛应用。其中,已经开发出几类基于古核素-3 的 GEVIs,并在各种分子成像研究中证明了自己的前景。为了扩大这类 GEVI 的应用范围,需要新的变体,其吸收带最大值向第一个生物窗口移动,并增强荧光信号。在这里,我们整合了计算和实验策略,揭示了区分基于弓形视蛋白-3 的远红光 GEVIs(Archers,在之前的研究中通过定向进化获得)和荧光信号极弱的野生型弓形视蛋白-3 的结构因素,旨在将获得的信息用于后续的合理设计。我们发现,稳定蛋白质的某种构象可以增强荧光,而稳定构象又可以通过调整两个可滴定残基的 pKa 值来实现。通过在野生型原核视蛋白-3中引入突变并检测荧光信号的增强情况,我们进一步证实了这些发现。最后,我们提出了一种合理的设计方案,并提出了以前未知的阿彻变体,它们具有红移吸收带(λmax 高达 640 纳米)和电位依赖性明亮荧光(量子产率高达 0.97%)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Rational Design of Far-Red Archaerhodopsin-3-Based Fluorescent Genetically Encoded Voltage Indicators: from Elucidation of the Fluorescence Mechanism in Archers to Novel Red-Shifted Variants
Genetically encoded voltage indicators (GEVIs) have found wide applications as molecular tools for visualization of changes in cell membrane potential. Among others, several classes of archaerhodopsin-3-based GEVIs have been developed and have proved themselves promising in various molecular imaging studies. To expand the application range for this type of GEVIs, new variants with absorption band maxima shifted toward the first biological window and enhanced fluorescence signal are required. Here, we integrate computational and experimental strategies to reveal structural factors that distinguish far-red bright archaerhodopsin-3-based GEVIs, Archers, obtained by directed evolution in a previous study (McIsaac et al., PNAS, 2014) and the wild-type archaerhodopsin-3 with an extremely dim fluorescence signal, aiming to use the obtained information in subsequent rational design. We found that the fluorescence can be enhanced by stabilization of a certain conformation of the protein, which, in turn, can be achieved by tuning the pKa value of two titratable residues. These findings were supported further by introducing mutations into wild-type archeorhodopsin-3 and detecting the enhancement of the fluorescence signal. Finally, we came up with a rational design and proposed previously unknown Archers variants with red-shifted absorption bands (λmax up to 640 nm) and potential-dependent bright fluorescence (quantum yield up to 0.97%).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
3.70
自引率
0.00%
发文量
0
期刊介绍:
ACS Physical Chemistry Au is an open access journal which publishes original fundamental and applied research on all aspects of physical chemistry. The journal publishes new and original experimental computational and theoretical research of interest to physical chemists biophysical chemists chemical physicists physicists material scientists and engineers. An essential criterion for acceptance is that the manuscript provides new physical insight or develops new tools and methods of general interest. Some major topical areas include:Molecules Clusters and Aerosols; Biophysics Biomaterials Liquids and Soft Matter; Energy Materials and Catalysis

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


