建立大规模血浆蛋白质组样品制备的质量控制指标
IF 4.6
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
由于样品制备工作流程的多重化和自动化,大规模血浆蛋白质组学研究已经发生了转变。然而,这些工作流程可能存在可重复性问题、缺乏标准化的质量控制(QC)指标,以及在液相色谱-串联质谱(LC-MS/MS)分析前评估变异等问题。在样品制备工作流程中纳入可靠的质量控制指标可确保更好的重现性、更低的检测变异以及更明智的故障排除决策。我们实验室使用串联质量标签(TMT)16-plex 批次(N = 58)对一组患者样本(N = 808)进行了血浆蛋白质组学研究。蛋白质组学工作流程包括蛋白质去除、蛋白质消化、TMT 标记和分馏。我们创建了五种质控样品类型(QCstd、QCdig、QCpool、QCTMT 和 QCBSA),用于测量最终 LC-MS/MS 分析前样品制备的性能。我们根据不同质控样品步骤的数据,测量了蛋白质组工作流程中各个样品制备步骤的 <10% CV。为样品制备步骤的质量控制建立了可靠的衡量标准,从而提高了后续 LC-MS/MS 分析中制备样品的可信度。这项研究还为标准化质量控制指标提供了建议,有助于未来大规模队列样品制备工作流程的开展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
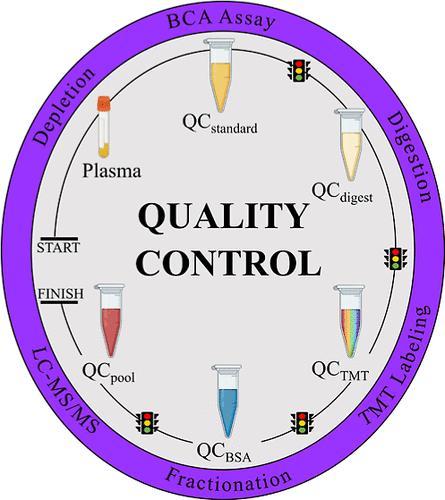
Establishing Quality Control Metrics for Large-Scale Plasma Proteomic Sample Preparation
Large-scale plasma proteomics studies have been transformed due to the multiplexing and automation of sample preparation workflows. However, these workflows can suffer from reproducibility issues, a lack of standardized quality control (QC) metrics, and the assessment of variation before liquid chromatography–tandem mass spectrometry (LC–MS/MS) analysis. The incorporation of robust QC metrics in sample preparation workflows ensures better reproducibility, lower assay variation, and better-informed decisions for troubleshooting. Our laboratory conducted a plasma proteomics study of a cohort of patient samples (N = 808) using tandem mass tag (TMT) 16-plex batches (N = 58). The proteomic workflow consisted of protein depletion, protein digestion, TMT labeling, and fractionation. Five QC sample types (QCstd, QCdig, QCpool, QCTMT, and QCBSA) were created to measure the performance of sample preparation prior to the final LC–MS/MS analysis. We measured <10% CV for individual sample preparation steps in the proteomic workflow based on data from various QC sample steps. The establishment of robust measures for QC of sample preparation steps allowed for greater confidence in prepared samples for subsequent LC–MS/MS analysis. This study also provides recommendations for standardized QC metrics that can assist with future large-scale cohort sample preparation workflows.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Measurement Science Au
化学计量学-
CiteScore
5.20
自引率
0.00%
发文量
0
期刊介绍:
ACS Measurement Science Au is an open access journal that publishes experimental computational or theoretical research in all areas of chemical measurement science. Short letters comprehensive articles reviews and perspectives are welcome on topics that report on any phase of analytical operations including sampling measurement and data analysis. This includes:Chemical Reactions and SelectivityChemometrics and Data ProcessingElectrochemistryElemental and Molecular CharacterizationImagingInstrumentationMass SpectrometryMicroscale and Nanoscale systemsOmics (Genomics Proteomics Metabonomics Metabolomics and Bioinformatics)Sensors and Sensing (Biosensors Chemical Sensors Gas Sensors Intracellular Sensors Single-Molecule Sensors Cell Chips Arrays Microfluidic Devices)SeparationsSpectroscopySurface analysisPapers dealing with established methods need to offer a significantly improved original application of the method.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


