不断发展的转移性尿路上皮癌治疗方法
IF 12.1
1区 医学
Q1 UROLOGY & NEPHROLOGY
引用次数: 0
摘要
顺铂化疗是目前治疗转移性尿路上皮癌(mUC)患者的一线标准疗法;然而,多达50%的患者不符合顺铂治疗条件,因此有必要选择其他治疗方案。免疫检查点抑制剂已被证明对不符合顺铂治疗条件的患者有效。然而,尽管在一线治疗方面取得了进展,但预后仍然很差,而且在选择最佳疗法、治疗顺序和联合方案方面仍然存在挑战。与单纯最佳支持治疗相比,使用阿维列单抗进行维持治疗可改善铂反应性mUC患者的总生存期(OS)和无进展生存期(PFS)。抗体药物共轭物和成纤维细胞生长因子受体(FGFR)抑制剂的靶向治疗已在部分患者中显示出前景,尤其是在铂类化疗后病情仍有进展的转移性疾病患者中。在2023年举行的欧洲肿瘤内科学会大会上,EV-302/KEYNOTE-A39和CheckMate 901两项III期试验取得了突破性成果,这两项试验主要针对既往未经治疗的mUC。在前者中,与单独化疗相比,恩福单抗-维多汀和彭博利珠单抗的联合用药在OS、PFS和总反应率方面均有显著改善;与单独化疗相比,尼夫单抗与吉西他滨-顺铂化疗的联合用药在中位OS、PFS和总反应率方面均有显著延长。此外,在既往接受过免疫检查点抑制剂治疗的 mUC 和表皮生长因子受体(FGFR)改变患者中,erdafitinib疗法的OS明显长于化疗。这篇关于mUC目前治疗情况的全面总结结合了临床试验证据和对目前正在研究的药物的讨论,为临床决策和了解未来的治疗方法提供了支持。本文章由计算机程序翻译,如有差异,请以英文原文为准。

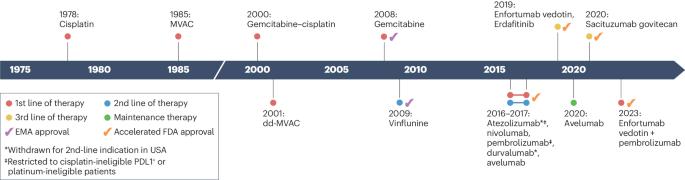
The evolving treatment landscape of metastatic urothelial cancer
Cisplatin-based chemotherapy is currently the first-line standard of care for patients with metastatic urothelial cancer (mUC); however, up to 50% of patients are ineligible for cisplatin, necessitating alternative treatment options. Immune checkpoint inhibitors have been shown to be effective in cisplatin-ineligible patients. However, despite advances in the first-line setting, the prognosis remains poor, and challenges persist in selecting optimal therapies, treatment sequences and combination regimens. Maintenance therapy with avelumab revealed improved overall (OS) and progression-free survival (PFS) compared with best supportive care alone in patients with platinum-responsive mUC. Antibody–drug conjugates and targeted therapy with fibroblast growth factor receptor (FGFR) inhibitors have shown promise in selected patients, particularly in patients with metastatic disease that has progressed despite platinum-based chemotherapy. At the European Society of Medical Oncology Congress in 2023, groundbreaking results were presented from two phase III trials, EV-302/KEYNOTE-A39 and CheckMate 901, focusing on previously untreated mUC. In the former, the combination of enfortumab vedotin and pembrolizumab showed significant improvements in OS, PFS and overall response rate compared with chemotherapy alone; the combination of nivolumab with gemcitabine–cisplatin chemotherapy demonstrated a significant extension in median OS, PFS and overall response rate compared with chemotherapy alone. In addition, erdafitinib therapy resulted in significantly longer OS than chemotherapy among patients with mUC and FGFR alterations after previous treatment with immune checkpoint inhibitors. This comprehensive summary of the current treatment landscape for mUC incorporates clinical trial evidence and discussion of agents that are currently under investigation to provide support for clinical decision making and understanding of future therapeutic approaches. Urothelial cancer is the tenth most diagnosed cancer worldwide, and between 5% and 10% of patients have metastatic disease at diagnosis. Furthermore, up to 50% of patients are ineligible for cisplatin, the first-line treatment option, and require alternative options. In this comprehensive Review, the authors discuss the current and future therapeutic options for advanced urothelial cancer, including chemotherapeutics, targeted therapies and antibody–drug conjugates, and consider how these agents might change patient management.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Reviews Urology
医学-泌尿学与肾脏学
CiteScore
12.50
自引率
2.60%
发文量
123
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Urology is part of the Nature Reviews portfolio of journals.Nature Reviews' basic, translational and clinical content is written by internationally renowned basic and clinical academics and researchers. This journal targeted readers in the biological and medical sciences, from the postgraduate level upwards, aiming to be accessible to professionals in any biological or medical discipline.
The journal features authoritative In-depth Reviews providing up-to-date information on topics within a field's history and development. Perspectives, News & Views articles, and the Research Highlights section offer topical discussions and opinions, filtering primary research from various medical journals.
Covering a wide range of subjects, including andrology, urologic oncology, and imaging, Nature Reviews provides valuable insights for practitioners, researchers, and academics within urology and related fields.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


