线粒体转移通过有丝分裂介导内皮细胞移植
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
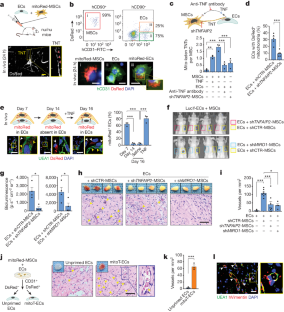
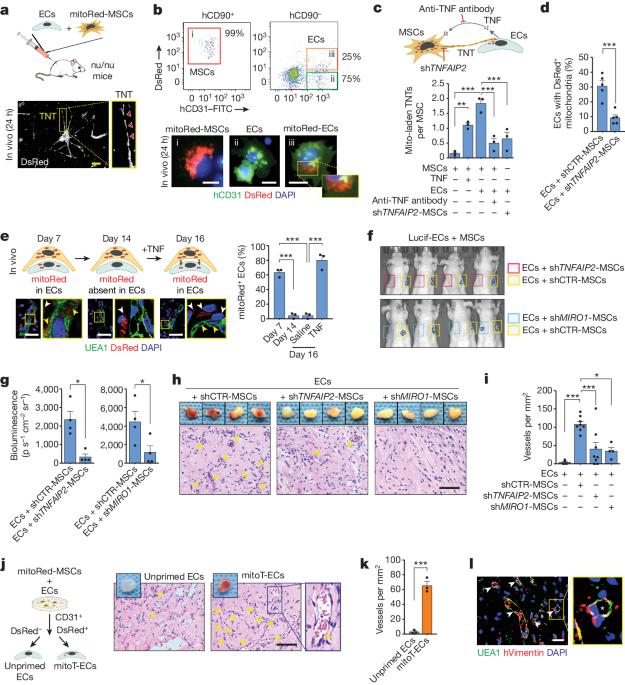
严重肢体缺血和心肌梗死等缺血性疾病影响着全球数百万人1。移植内皮细胞(ECs)是血管医学中一种前景广阔的疗法,但移植ECs通常需要同时移植间充质基质细胞(MSCs)等血管周围支持细胞,这使得临床实施变得复杂2,3。间充质干细胞促进心肌细胞移植的机制仍不明确。我们在这里发现,在细胞应激状态下,间充质干细胞会通过隧道式纳米管将线粒体转移到心肌细胞,而阻断这种转移会影响心肌细胞的移植。我们设计了一种人工移植线粒体的策略,可短暂增强心肌细胞的生物能,使其在缺血组织中形成功能性血管,而无需间充质干细胞的支持。值得注意的是,外源性线粒体不会整合到内源性心肌线粒体池中,而是在内化后引发有丝分裂。移植的线粒体与自噬体共定位,PINK1-Parkin通路的消减否定了ECs增强的移植能力。我们的研究结果揭示了间充质细胞和内皮细胞间线粒体转移效应的机制,为血管细胞治疗提供了一种新方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Mitochondrial transfer mediates endothelial cell engraftment through mitophagy
Ischaemic diseases such as critical limb ischaemia and myocardial infarction affect millions of people worldwide1. Transplanting endothelial cells (ECs) is a promising therapy in vascular medicine, but engrafting ECs typically necessitates co-transplanting perivascular supporting cells such as mesenchymal stromal cells (MSCs), which makes clinical implementation complicated2,3. The mechanisms that enable MSCs to facilitate EC engraftment remain elusive. Here we show that, under cellular stress, MSCs transfer mitochondria to ECs through tunnelling nanotubes, and that blocking this transfer impairs EC engraftment. We devised a strategy to artificially transplant mitochondria, transiently enhancing EC bioenergetics and enabling them to form functional vessels in ischaemic tissues without the support of MSCs. Notably, exogenous mitochondria did not integrate into the endogenous EC mitochondrial pool, but triggered mitophagy after internalization. Transplanted mitochondria co-localized with autophagosomes, and ablation of the PINK1–Parkin pathway negated the enhanced engraftment ability of ECs. Our findings reveal a mechanism that underlies the effects of mitochondrial transfer between mesenchymal and endothelial cells, and offer potential for a new approach for vascular cell therapy. Under stressful conditions, mesenchymal stromal cells transfer mitochondria to endothelial cells through tunnelling nanotubes, and artificially transplanting mitochondria into endothelial cells improves the ability of these cells to engraft and to revascularize ischaemic tissues.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


