组氨酸激酶介导的难辨梭状芽孢杆菌抗万古霉素操作子的交叉调节
IF 2.6
2区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
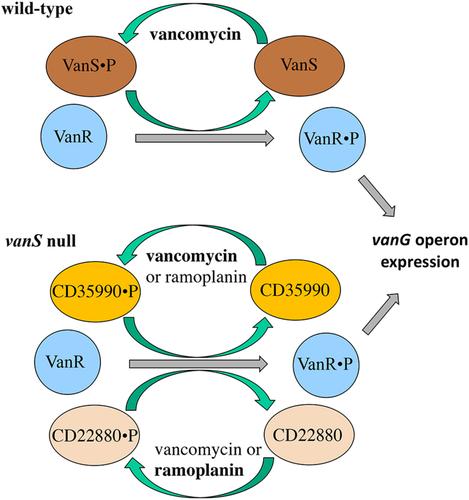
二肽 D-Ala-D-Ala 是肽聚糖的重要组成部分,也是万古霉素的靶标。大多数艰难梭菌菌株都具有负责合成 D-Ala-D-Ser 的 vanG 操作子,D-Ala-D-Ser 可以取代肽聚糖中的 D-Ala-D-Ala。艰难梭菌的 vanG 操作子由双组分系统 VanRS 调节,但万古霉素并不能充分诱导其产生抗药性。令人惊讶的是,在缺乏 VanS 组氨酸激酶(HK)的情况下,万古霉素仍能诱导 vanG 操作子,而另一种抗生素 ramoplanin 也能以 VanR 依赖性方式诱导 vanG 操作子。这表明 VanR 受另一种 HK 或激酶的交叉调节,这些 HK 或激酶在某些脂质 II 靶向抗生素存在时被激活。我们确定这些 HK 为 CD35990 和 CD22880。然而,这两个HKs的突变并不影响野生型细胞中vanG操作子的调控,这表明完整的VanS可以防止VanR被非识别HKs交叉激活。在没有 VanS、CD35990 和 CD22880 的情况下,VanR 的过度生产导致 vanG 操作子的高表达,这表明 VanR 有可能至少利用另外一种磷酸盐供体来激活。通过 RNA-Seq 确定了 CD35990 和 CD22880 在万古霉素或雷莫普兰存在时介导调控的候选靶标。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Histidine kinase-mediated cross-regulation of the vancomycin-resistance operon in Clostridioides difficile
The dipeptide D-Ala-D-Ala is an essential component of peptidoglycan and the target of vancomycin. Most Clostridioides difficile strains possess the vanG operon responsible for the synthesis of D-Ala-D-Ser, which can replace D-Ala-D-Ala in peptidoglycan. The C. difficile vanG operon is regulated by a two-component system, VanRS, but is not induced sufficiently by vancomycin to confer resistance to this antibiotic. Surprisingly, in the absence of the VanS histidine kinase (HK), the vanG operon is still induced by vancomycin and also by another antibiotic, ramoplanin, in a VanR-dependent manner. This suggested the cross-regulation of VanR by another HK or kinases that are activated in the presence of certain lipid II-targeting antibiotics. We identified these HKs as CD35990 and CD22880. However, mutations in either or both HKs did not affect the regulation of the vanG operon in wild-type cells suggesting that intact VanS prevents the cross-activation of VanR by non-cognate HKs. Overproduction of VanR in the absence of VanS, CD35990, and CD22880 led to high expression of the vanG operon indicating that VanR can potentially utilize at least one more phosphate donor for its activation. Candidate targets of CD35990- and CD22880-mediated regulation in the presence of vancomycin or ramoplanin were identified by RNA-Seq.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Microbiology
生物-生化与分子生物学
CiteScore
7.20
自引率
5.60%
发文量
132
审稿时长
1.7 months
期刊介绍:
Molecular Microbiology, the leading primary journal in the microbial sciences, publishes molecular studies of Bacteria, Archaea, eukaryotic microorganisms, and their viruses.
Research papers should lead to a deeper understanding of the molecular principles underlying basic physiological processes or mechanisms. Appropriate topics include gene expression and regulation, pathogenicity and virulence, physiology and metabolism, synthesis of macromolecules (proteins, nucleic acids, lipids, polysaccharides, etc), cell biology and subcellular organization, membrane biogenesis and function, traffic and transport, cell-cell communication and signalling pathways, evolution and gene transfer. Articles focused on host responses (cellular or immunological) to pathogens or on microbial ecology should be directed to our sister journals Cellular Microbiology and Environmental Microbiology, respectively.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


