致癌 Kras 通过将脉冲式 ERK 激活转化为持续式 ERK 激活,诱导时空特异性组织变形
IF 17.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
组织再生和维护依赖于干细胞的协调行为。这种协调可因致癌基因突变而受损,导致癌症。然而,目前还不清楚致癌基因如何扰乱干细胞的协调行为,从而破坏组织。在这里,我们利用眼内成像技术研究致癌基因Kras突变导致毛囊组织破坏的机制。通过纵向追踪活体小鼠的毛囊,我们发现 KrasG12D(一种可导致鳞状细胞癌的突变)以时空特异性的方式诱导上皮组织变形,并与异常的细胞分裂和迁移有关。我们利用报告小鼠捕捉单细胞水平的实时ERK信号动态,发现KrasG12D,而非密切相关的突变HrasG12V,能将干细胞中的ERK信号从脉冲式转变为持续式。最后,我们证明,中断持续的ERK信号可通过调节细胞迁移和分裂的特定特征,恢复KrasG12D诱导的组织变形。本文章由计算机程序翻译,如有差异,请以英文原文为准。
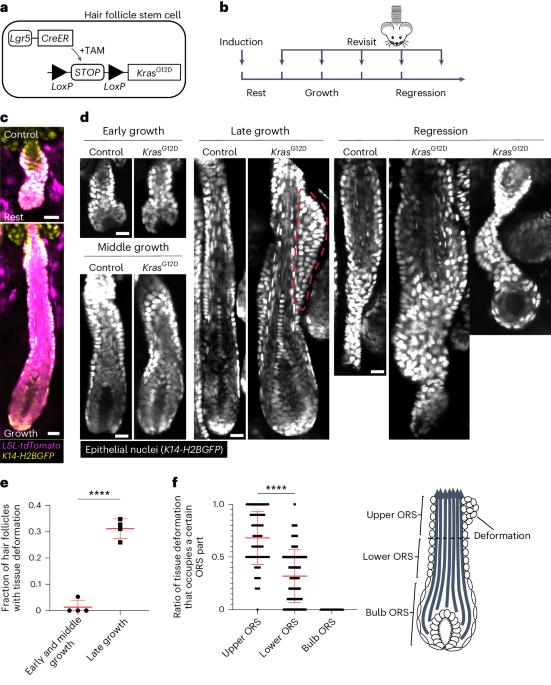
Oncogenic Kras induces spatiotemporally specific tissue deformation through converting pulsatile into sustained ERK activation
Tissue regeneration and maintenance rely on coordinated stem cell behaviours. This orchestration can be impaired by oncogenic mutations leading to cancer. However, it is largely unclear how oncogenes perturb stem cells’ orchestration to disrupt tissue. Here we used intravital imaging to investigate the mechanisms by which oncogenic Kras mutation causes tissue disruption in the hair follicle. Through longitudinally tracking hair follicles in live mice, we found that KrasG12D, a mutation that can lead to squamous cell carcinoma, induces epithelial tissue deformation in a spatiotemporally specific manner, linked with abnormal cell division and migration. Using a reporter mouse capture real-time ERK signal dynamics at the single-cell level, we discovered that KrasG12D, but not a closely related mutation HrasG12V, converts ERK signal in stem cells from pulsatile to sustained. Finally, we demonstrated that interrupting sustained ERK signal reverts KrasG12D-induced tissue deformation through modulating specific features of cell migration and division. Xin et al. show, through intravital imaging, that KrasG12D induces epithelial tissue deformation in a spatiotemporally specific manner by converting the pulsatile ERK signal fluctuation in stem cells into sustained activation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


