靶向蛋白质降解:从机制到临床
IF 81.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
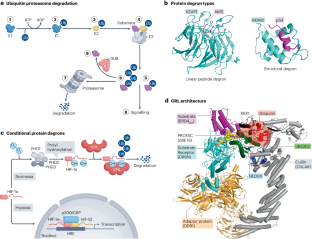
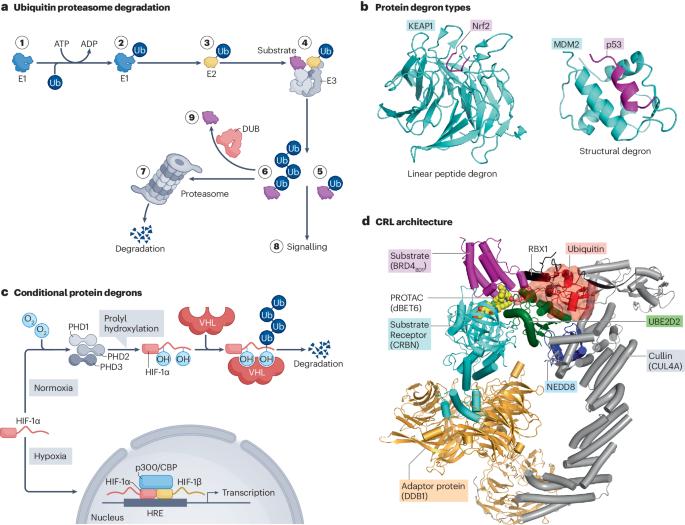
靶向蛋白质降解是指使用小分子诱导蛋白质的选择性降解。在最常见的形式中,这种降解是通过配体介导的泛素 E3 连接酶(细胞的主要废物处理机)与相关蛋白质靶标之间的新相互作用来实现的,从而导致泛素化和随后的蛋白酶体降解。人们对偶然发现的降解剂的生物学和机理认识取得了显著进展。这种深入的了解和新颖的化学方法不仅为靶向蛋白质降解提供了临床概念验证,而且还促使该领域迅速发展,目前已有数十种在研药物正在进行临床试验。目前正在广泛探索两类不同的蛋白质降解疗法:双功能 PROTACs 和分子胶降解剂,这两种疗法都有其独特的优势和挑战。在此,我们回顾了目前靶向蛋白质降解方法的现状,以及它们在生物过程中的相似之处。我们还概述了正在进行的新型降解剂临床探索,并对该领域可能的发展方向提出了一些看法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Targeted protein degradation: from mechanisms to clinic
Targeted protein degradation refers to the use of small molecules to induce the selective degradation of proteins. In its most common form, this degradation is achieved through ligand-mediated neo-interactions between ubiquitin E3 ligases — the principal waste disposal machines of a cell — and the protein targets of interest, resulting in ubiquitylation and subsequent proteasomal degradation. Notable advances have been made in biological and mechanistic understanding of serendipitously discovered degraders. This improved understanding and novel chemistry has not only provided clinical proof of concept for targeted protein degradation but has also led to rapid growth of the field, with dozens of investigational drugs in active clinical trials. Two distinct classes of protein degradation therapeutics are being widely explored: bifunctional PROTACs and molecular glue degraders, both of which have their unique advantages and challenges. Here, we review the current landscape of targeted protein degradation approaches and how they have parallels in biological processes. We also outline the ongoing clinical exploration of novel degraders and provide some perspectives on the directions the field might take. This article reviews the current landscape of targeted protein degradation approaches and how they have parallels in biological processes. The authors also outline the ongoing clinical exploration of novel degraders and provide some perspectives on the directions the field might take.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
173.60
自引率
0.50%
发文量
118
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Molecular Cell Biology is a prestigious journal that aims to be the primary source of reviews and commentaries for the scientific communities it serves. The journal strives to publish articles that are authoritative, accessible, and enriched with easily understandable figures, tables, and other display items. The goal is to provide an unparalleled service to authors, referees, and readers, and the journal works diligently to maximize the usefulness and impact of each article. Nature Reviews Molecular Cell Biology publishes a variety of article types, including Reviews, Perspectives, Comments, and Research Highlights, all of which are relevant to molecular and cell biologists. The journal's broad scope ensures that the articles it publishes reach the widest possible audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


