从 Lendenfeldia 属海绵中分离出 24-homoscalarane 酯类化合物
IF 1.3
4区 生物学
Q4 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
通过对一种被鉴定为 Lendenfeldia 物种的含藻海洋海绵进行化学筛选,分离出了两种 24-homoscalarane 酯类化合物,包括一种已知化合物 felixin A(1)和一种新的类似物 lendenfeldarane V(2)。通过单晶 X 射线衍射分析,本研究首次引用了之前研究中获得的 1 的结构。2 的结构是通过二维核磁共振实验和文献综述确定的。通过 DP4+ 计算和特定光学旋转,划定了 1 和 2 的绝对构型。Homoscalarane 1 对 MG63 人类骨肉瘤细胞具有细胞毒性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
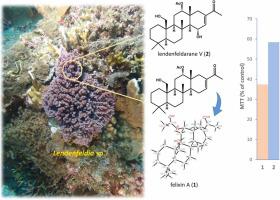
Isolation of 24-homoscalarane sesterterpenoids from a sponge of the genus Lendenfeldia
The chemical screening of an algae-containing marine sponge identified as Lendenfeldia species has isolated two 24-homoscalarane sesterterpenoids, including a known compound, felixin A (1) and a new analogue, lendenfeldarane V (2). The structure of 1, obtained in a previous study, was cited for the first time in this study via single-crystal X-ray diffraction analysis. The structure of 2 was ascertained via 2D NMR experiments and a literature review. The absolute configurations of 1 and 2 were delineated with DP4+ computation and specific optical rotation. Homoscalarane 1 exhibited cytotoxicity towards MG63 human osteosarcoma cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytochemistry Letters
生物-生化与分子生物学
CiteScore
3.00
自引率
11.80%
发文量
190
审稿时长
34 days
期刊介绍:
Phytochemistry Letters invites rapid communications on all aspects of natural product research including:
• Structural elucidation of natural products
• Analytical evaluation of herbal medicines
• Clinical efficacy, safety and pharmacovigilance of herbal medicines
• Natural product biosynthesis
• Natural product synthesis and chemical modification
• Natural product metabolism
• Chemical ecology
• Biotechnology
• Bioassay-guided isolation
• Pharmacognosy
• Pharmacology of natural products
• Metabolomics
• Ethnobotany and traditional usage
• Genetics of natural products
Manuscripts that detail the isolation of just one new compound are not substantial enough to be sent out of review and are out of scope. Furthermore, where pharmacology has been performed on one new compound to increase the amount of novel data, the pharmacology must be substantial and/or related to the medicinal use of the producing organism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


