阴极表面积对柠檬酸盐浴沉积的 Co-W 涂层的电沉积速率、成分和显微硬度的影响
IF 0.9
Q3 Engineering
Surface Engineering and Applied Electrochemistry
Pub Date : 2024-04-26
DOI:10.3103/S1068375524020042
引用次数: 0
摘要
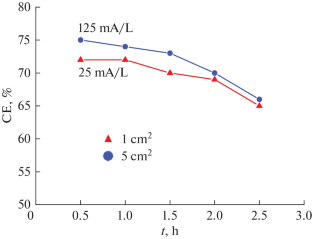
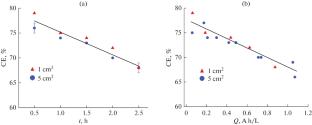
摘要 在这里,我们以柠檬酸盐浴中的 Co-W 涂层的电静电沉积为例,通过实验证明,当利用在实验室条件下观察到的沉积速率、所得涂层的成分和特性(微硬度)方面的结果,在更大(工业)规模上开发这种类型的电沉积工艺时,浴槽体积必须与阴极面积的增加成比例。在这种情况下,电解液上的电流负荷(用体积电流密度定量表示)不会增加。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Effect of Cathode Surface Area on the Electrodeposition Rate, Composition, and Microhardness of Co–W Coatings Deposited from a Citrate Bath
Here, by the example of galvanostatic electrodeposition of Co–W coatings from a citrate bath, we demonstrate experimentally that when using the results on the deposition rate and the composition and properties (microhardness) of resulting coatings observed under laboratory conditions to develop this type of an electrodeposition process on a larger (industrial) scale the bath volume must be scaled in proportion to the increase in the cathode area. In this case, the current loading on the electrolyte, which is quantitatively expressed as the volume current density, does not increase.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Surface Engineering and Applied Electrochemistry
Engineering-Industrial and Manufacturing Engineering
CiteScore
1.60
自引率
22.20%
发文量
54
期刊介绍:
Surface Engineering and Applied Electrochemistry is a journal that publishes original and review articles on theory and applications of electroerosion and electrochemical methods for the treatment of materials; physical and chemical methods for the preparation of macro-, micro-, and nanomaterials and their properties; electrical processes in engineering, chemistry, and methods for the processing of biological products and food; and application electromagnetic fields in biological systems.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


