MG132诱导的蛋白毒性应激对αB-结晶素和desmin磷酸化和O-GlcNA酰化及其向细胞骨架分化的影响
IF 3.3
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
小热休克蛋白被认为是蛋白稳态失效时的第一道防线。其中,αB-结晶蛋白在横纹肌中表达,它与 Desmin 中间丝相互作用以稳定这些中间丝,从而维持细胞骨架的完整性和肌肉功能。Desmin 是肌肉健康的关键因素;因此,αB-结晶素靶向 Desmin 至关重要,尤其是在压力条件下。αB-结晶素具有磷酸化和 O-GlcNAcylated 两种功能。在各种应激条件下,αB-结晶素的磷酸化都会增加,这与αB-结晶素被用于保护细胞骨架有关。然而,磷酸化作为细胞骨架转运的独特信号仍存在争议;事实上,O-GlcNAcylation 也被认为参与其中。因此,要深入了解αB-结晶素的功能如何受到翻译后修饰的精细调控,还存在一些空白。在本文中,我们旨在确定磷酸化和/或O-GlcNAcylation是否参与αB-结晶素在蛋白酶体抑制诱导的蛋白毒性应激中靶向细胞骨架的过程。我们证实,蛋白毒性导致αB-结晶素的磷酸化和O-GlcNAcylation模式发生变化,二者根据蛋白质亚组分的不同呈现出动态的相互作用。重要的是,这两种翻译后修饰的时空变化与αB-结晶素向细胞骨架的转移相关。相反,我们没有检测到 desmin 磷酸化和 O-GlcNAcylation 的任何变化。综上所述,这些数据有力地证明了αB-结晶素磷酸化/O-GlcNA酰化之间的相互作用,而不是desmin的变化,是其向细胞骨架转位的关键调节因子,从而保护其免受应激。本文章由计算机程序翻译,如有差异,请以英文原文为准。
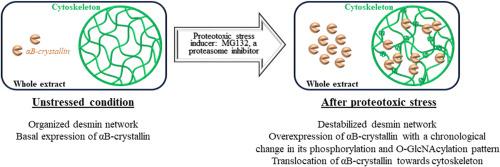
Impact of MG132 induced-proteotoxic stress on αB-crystallin and desmin phosphorylation and O-GlcNAcylation and their partition towards cytoskeleton
Small Heat Shock Proteins are considered as the first line of defense when proteostasis fails. Among them, αB-crystallin is expressed in striated muscles in which it interacts with desmin intermediate filaments to stabilize them, maintaining cytoskeleton's integrity and muscular functionalities. Desmin is a key actor for muscle health; its targeting by αB-crystallin is thus crucial, especially in stress conditions.
αB-crystallin is phosphorylated and O-GlcNAcylated. Its phosphorylation increases consecutively to various stresses, correlated with its recruitment for cytoskeleton's safeguarding. However, phosphorylation as unique signal for cytoskeleton translocation remains controversial; indeed, O-GlcNAcylation was also proposed to be involved. Thus, there are still some gaps for a deeper comprehension of how αB-crystallin functions are finely regulated by post-translational modifications. Furthermore, desmin also bears both post-translational modifications; while desmin phosphorylation is closely linked to desmin intermediates filaments turnover, it is unclear whereas its O-GlcNAcylation could impact its proper function.
In the herein paper, we aim at identifying whether phosphorylation and/or O-GlcNAcylation are involved in αB-crystallin targeting towards cytoskeleton in proteotoxic stress induced by proteasome inhibition in C2C12 myotubes. We demonstrated that proteotoxicity led to αB-crystallin's phosphorylation and O-GlcNAcylation patterns changes, both presenting a dynamic interplay depending on protein subfraction. Importantly, both post-translational modifications showed a spatio-temporal variation correlated with αB-crystallin translocation towards cytoskeleton. In contrast, we did not detect any change of desmin phosphorylation and O-GlcNAcylation. All together, these data strongly support that αB-crystallin phosphorylation/O-GlcNAcylation interplay rather than changes on desmin is a key regulator for its cytoskeleton translocation, preserving it towards stress.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochimie
生物-生化与分子生物学
CiteScore
7.20
自引率
2.60%
发文量
219
审稿时长
40 days
期刊介绍:
Biochimie publishes original research articles, short communications, review articles, graphical reviews, mini-reviews, and hypotheses in the broad areas of biology, including biochemistry, enzymology, molecular and cell biology, metabolic regulation, genetics, immunology, microbiology, structural biology, genomics, proteomics, and molecular mechanisms of disease. Biochimie publishes exclusively in English.
Articles are subject to peer review, and must satisfy the requirements of originality, high scientific integrity and general interest to a broad range of readers. Submissions that are judged to be of sound scientific and technical quality but do not fully satisfy the requirements for publication in Biochimie may benefit from a transfer service to a more suitable journal within the same subject area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


