幽门螺杆菌诱导的 LRP8 表达增强,通过促进 β-Catenin 核转位推动胃癌进展。
IF 11.4
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
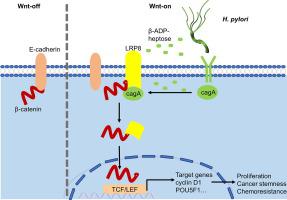

Enhanced LRP8 expression induced by Helicobacter pylori drives gastric cancer progression by facilitating β-Catenin nuclear translocation
Introduction
Helicobacter pylori (H. pylori) infection has been associated with gastric carcinogenesis. However, the precise involvement of LRP8, the low-density lipoprotein receptor-related protein 8, in H. pylori pathogenesis and gastric cancer (GC) remains poorly understood.
Objectives
To investigate the potential role of LRP8 in H. pylori infection and gastric carcinogenesis.
Methods
Three-dimensional human-derived gastric organoids (hGO) and gastric cancer organoids (hGCO) were synthesized from the tissues obtained from human donors. In this work, multi-omics combined with in vivo and in vitro studies were conducted to investigate the potential involvement of LRP8 in H. pylori-induced GC.
Results
We found that H. pylori infection significantly upregulated the expression of LRP8 in human GC tissues, cells, organoids, and mouse gastric mucous. In particular, LRP8 exhibited a distinct enrichment in cancer stem cells (CSC). Functionally, silencing of LRP8 affected the formation and proliferation of tumor spheroids, while increased expression of LRP8 was associated with increased proliferation and stemness of GC cells and organoids. Mechanistically, LRP8 promotes the binding of E-cadherin to β-catenin, thereby promoting nuclear translocation and transcriptional activity of β-catenin. Furthermore, LRP8 interacts with the cytotoxin-associated gene A (CagA) to form the CagA/LRP8/β-catenin complex. This complex further amplifies H. pylori-induced β-catenin nuclear translocation, leading to increased transcription of inflammatory factors and CSC markers. Clinical analysis demonstrated that abnormal overexpression of LRP8 is correlated with a poor prognosis and resistance to 5-Fluorouracil in patients with GC.
Conclusion
Our findings provide valuable information on the molecular intricacies of H. pylori-induced gastric carcinogenesis, offering potential therapeutic targets and prognostic markers for GC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


