鉴定结核分枝杆菌中的 1-酰基-甘油-3-磷酸酰基转移酶--一种参与三酰甘油生物合成的关键酶
IF 2.6
2区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
由休眠结核分枝杆菌(Mtb)引起的潜伏结核病通过在社区内潜伏未确诊的感染而对全球健康构成威胁。休眠分枝杆菌在表型上对抗生素具有耐受性,它利用从巨噬细胞脂滴中获得的脂肪酸积累三酰甘油(TAG)。TAG 对分枝杆菌至关重要,是潜伏期的细胞包膜成分和能量储存库。TAG 的合成是通过甘油-3-磷酸的连续酰化进行的,其中第二步酰化由酰基甘油-3-磷酸酰基转移酶(AGPAT)催化,从而产生磷脂酸(PA),这是合成 TAG 和各种磷脂的前体。在这里,我们对 Rv3816c 编码的 Mtb 的推定酰基转移酶进行了鉴定。我们发现 Rv3816c 具有 AGPAT 的所有四个特征基序,是一种膜结合酶,具有 1-酰基甘油-3-磷酸酰基转移酶的功能。该酶可将链长为 16 或 18 的单不饱和脂肪酰辅酶 A 的酰基转移至酰基甘油-3-磷酸(LPA),从而产生 PA。Rv3816c 对大肠杆菌 PlsC 突变体的体内互补证实了它具有 AGPAT 的功能。其活性位点突变体 H43A 和 D48A 不能在体外将酰基转移到 LPA,也不能挽救大肠杆菌 PlsC 突变体在体内的生长缺陷。将 Rv3816c 鉴定为 AGPAT 并将其特性与其他 AGPAT 同源物进行比较,不仅是朝着了解分枝杆菌中 TAG 生物合成的方向迈出了一步,而且有可能将其作为药物靶点进行研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
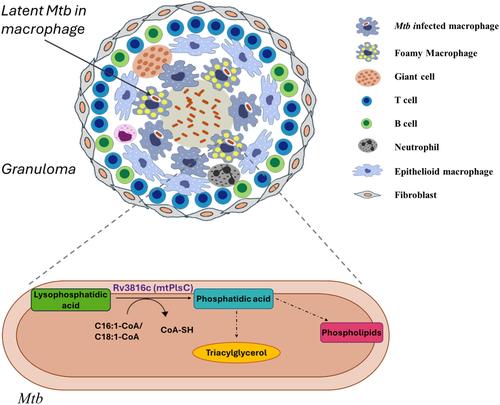
Identification of a 1-acyl-glycerol-3-phosphate acyltransferase from Mycobacterium tuberculosis, a key enzyme involved in triacylglycerol biosynthesis
Latent tuberculosis, caused by dormant Mycobacterium tuberculosis (Mtb), poses a threat to global health through the incubation of undiagnosed infections within the community. Dormant Mtb, which is phenotypically tolerant to antibiotics, accumulates triacylglycerol (TAG) utilizing fatty acids obtained from macrophage lipid droplets. TAG is vital to mycobacteria, serving as a cell envelope component and energy reservoir during latency. TAG synthesis occurs by sequential acylation of glycerol-3-phosphate, wherein the second acylation step is catalyzed by acylglycerol-3-phosphate acyltransferase (AGPAT), resulting in the production of phosphatidic acid (PA), a precursor for the synthesis of TAG and various phospholipids. Here, we have characterized a putative acyltransferase of Mtb encoded by Rv3816c. We found that Rv3816c has all four characteristic motifs of AGPAT, exists as a membrane-bound enzyme, and functions as 1-acylglycerol-3-phosphate acyltransferase. The enzyme could transfer the acyl group to acylglycerol-3-phosphate (LPA) from monounsaturated fatty acyl-coenzyme A of chain length 16 or 18 to produce PA. Complementation of Escherichia coli PlsC mutant in vivo by Rv3816c confirmed that it functions as AGPAT. Its active site mutants, H43A and D48A, were incapable of transferring the acyl group to LPA in vitro and were not able to rescue the growth defect of E. coli PlsC mutant in vivo. Identifying Rv3816c as AGPAT and comparing its properties with other AGPAT homologs is not only a step toward understanding the TAG biosynthesis in mycobacteria but has the potential to explore it as a drug target.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Microbiology
生物-生化与分子生物学
CiteScore
7.20
自引率
5.60%
发文量
132
审稿时长
1.7 months
期刊介绍:
Molecular Microbiology, the leading primary journal in the microbial sciences, publishes molecular studies of Bacteria, Archaea, eukaryotic microorganisms, and their viruses.
Research papers should lead to a deeper understanding of the molecular principles underlying basic physiological processes or mechanisms. Appropriate topics include gene expression and regulation, pathogenicity and virulence, physiology and metabolism, synthesis of macromolecules (proteins, nucleic acids, lipids, polysaccharides, etc), cell biology and subcellular organization, membrane biogenesis and function, traffic and transport, cell-cell communication and signalling pathways, evolution and gene transfer. Articles focused on host responses (cellular or immunological) to pathogens or on microbial ecology should be directed to our sister journals Cellular Microbiology and Environmental Microbiology, respectively.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


