TEMPO 介导的芳胺与多氟化醇的羟基氟烷基化反应
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
通过自由基触发的 C(sp2)-H/C(sp3)-H 脱氢交叉偶联过程,开发了一种高效的 2,2,6,6-四甲基哌啶氧(TEMPO)介导的芳胺与多氟化醇的羟基氟烷基化反应。这一转化过程具有操作简单、原子经济性高、底物相容性广以及优异的区域选择性等特点,从而产生了一系列羟基氟烷基化芳胺衍生物。重要的是,这些合成产物被进一步用于通过细胞计数试剂盒-8 法评估其在癌细胞株中的抗肿瘤活性,结果表明一些化合物具有强效的抗增殖作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
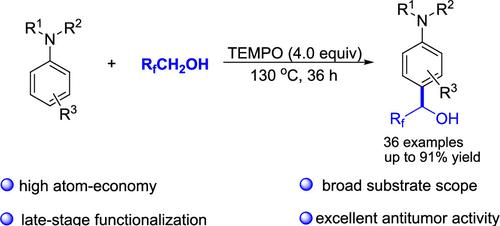
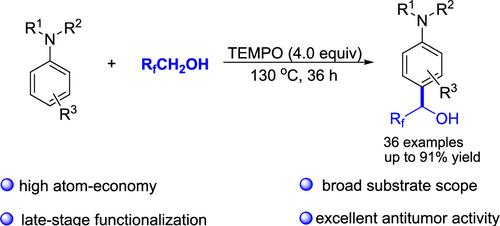
TEMPO-Mediated Dehydrogenative Hydroxyfluoroalkylation of Arylamines with Polyfluorinated Alcohols
An efficient 2,2,6,6-tetramethylpiperidinooxy (TEMPO)-mediated hydroxyfluoroalkylation of arylamines with polyfluorinated alcohols via a radical-triggered C(sp2)–H/C(sp3)–H dehydrogenative cross-coupling process was developed. This transformation features simple operation, high atom economy, broad substrate compatibility, and excellent regioselectivity, leading to a series of hydroxyfluoroalkylated arylamine derivatives. Importantly, these synthetic products were further used to evaluate the antitumor activity in cancer cell lines by Cell Counting Kit-8 assay and the outcomes indicated that some compounds show a potent antiproliferative effect.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


