Onabotulinum toxin A 可改善脊髓损伤后的神经源性逼尿肌过度活动:系统综述与荟萃分析
IF 2.1
4区 医学
Q3 CLINICAL NEUROLOGY
引用次数: 0
摘要
研究设计系统综述和荟萃分析.目的本研究旨在评估奥那曲毒素 A(OBTX-A)治疗脊髓损伤(SCI)患者神经源性逼尿肌过度活动(NDO)的疗效和安全性.研究地点伊朗.方法纳入了截至 2022 年 9 月 6 日在 PubMed/MEDLINE、Embase、Scopus 和 Web of Science 数据库中收录的所有涉及奥那曲毒素 A 治疗脊髓损伤后 NDO 的临床试验和队列研究的相关文章。采用 Cochrane 标准对符合条件的研究进行质量评估。结果关于 OBTX-A 短期治疗后的总体疗效,每次排尿量(VV)(WMD = 118.8,95% CI:90.9-146.7,p < 0.01)、尿失禁生活质量(IQoL)(WMD = 24.3,95% CI:15.8-32.8, p <0.01)和最大膀胱容量(MCC)(WMD = 144.5, 95% CI: 132.3 to 156.7, p <0.01)显著增加,而储尿期最大逼尿肌压力(MDP)(WMD = -30.5, 95% CI: -35.9 to -25.1,p<0.01)显著降低。此外,与服用 200 单位剂量的安慰剂组相比,MCC 有明显增加(WMD = 113.5,95% CI:84.7 至 142.3,p < 0.01),MDP 有明显下降(WMD = -27.2,95% CI:-39.2 至 -15.1,p < 0.01)。尿路感染 (UTI)、血尿和自主神经反射障碍是最常见的副作用,发生率分别为 29.6%、14.8% 和 13.4%。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Onabotulinum toxin A improves neurogenic detrusor overactivity following spinal cord injury: a systematic review and meta-analysis
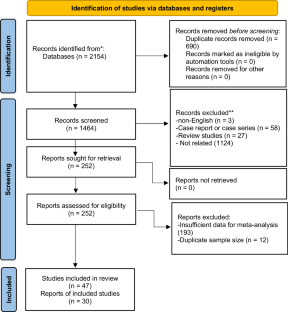
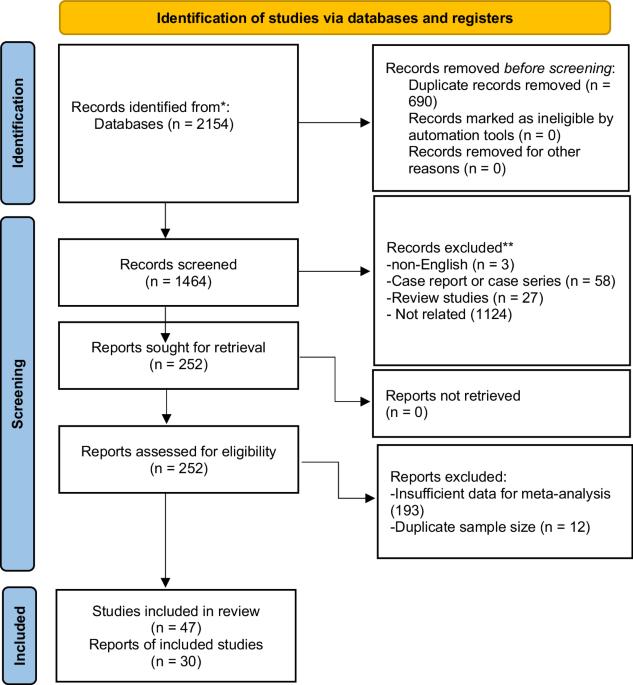
Systematic review and meta-analysis. The current study aimed to assess the efficacy and safety of Onabotulinum toxin A (OBTX-A) treatment for neurogenic detrusor overactivity (NDO) in spinal cord injury (SCI) patients. Iran. All relevant articles of clinical trials and cohort studies indexed in PubMed/MEDLINE, Embase, Scopus, and Web of Science databases up to September 6, 2022, that addressed OBTX-A treatment for NDO following SCI were included. The quality of eligible studies was evaluated using Cochrane criteria. Also, the weighted mean difference (WMD) was measured with a random-effect model. Regarding the overall efficacy after OBTX-A treatment in the short term, volume per void (VV) (WMD = 118.8, 95% CI: 90.9–146.7, p < 0.01), incontinence-quality of life (IQoL) (WMD = 24.3, 95% CI: 15.8–32.8, p < 0.01), and maximum cystometric capacity (MCC) (WMD = 144.5, 95% CI: 132.3 to 156.7, p < 0.01) significantly increased, while maximum detrusor pressure during storage (MDP) (WMD = –30.5, 95% CI: –35.9 to –25.1, p < 0.01) showed a significant decrease. Furthermore, compared to the placebo group at the 200-unit dose, there was a significant increase in MCC (WMD = 113.5, 95% CI: 84.7 to 142.3, p < 0.01) and a significant decrease in MDP (WMD = −27.2, 95% CI: −39.2 to −15.1, p < 0.01). Urinary tract infection (UTI), hematuria, and autonomic dysreflexia were the most common side effects, occurring at rates of 29.6%, 14.8%, and 13.4%, respectively. Our findings highlighted the effectiveness and safety of OBTX-A as a promising treatment of NDO following SCI.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Spinal cord
医学-临床神经学
CiteScore
4.50
自引率
9.10%
发文量
142
审稿时长
2 months
期刊介绍:
Spinal Cord is a specialised, international journal that has been publishing spinal cord related manuscripts since 1963. It appears monthly, online and in print, and accepts contributions on spinal cord anatomy, physiology, management of injury and disease, and the quality of life and life circumstances of people with a spinal cord injury. Spinal Cord is multi-disciplinary and publishes contributions across the entire spectrum of research ranging from basic science to applied clinical research. It focuses on high quality original research, systematic reviews and narrative reviews.
Spinal Cord''s sister journal Spinal Cord Series and Cases: Clinical Management in Spinal Cord Disorders publishes high quality case reports, small case series, pilot and retrospective studies perspectives, Pulse survey articles, Point-couterpoint articles, correspondences and book reviews. It specialises in material that addresses all aspects of life for persons with spinal cord injuries or disorders. For more information, please see the aims and scope of Spinal Cord Series and Cases.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


