催化甲烷分解产生碳中和氢气:综述
IF 15
2区 环境科学与生态学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
预计全球氢气需求量将从 2019 年的 7000 万吨增至 2030 年的 2 亿吨以上。甲烷分解是一种很有前景的制氢反应,同时还能合成有价值的碳纳米材料,应用于燃料电池技术、运输燃料和化学合成。在此,我们回顾了催化甲烷分解,重点是催化剂的开发、失活、再活化、再生和经济性。催化剂包括单金属、双金属和三金属化合物以及碳基化合物。焦炭沉积会导致催化剂失活。尽管研究取得了长足进步,但工业化仍处于早期阶段。本文章由计算机程序翻译,如有差异,请以英文原文为准。
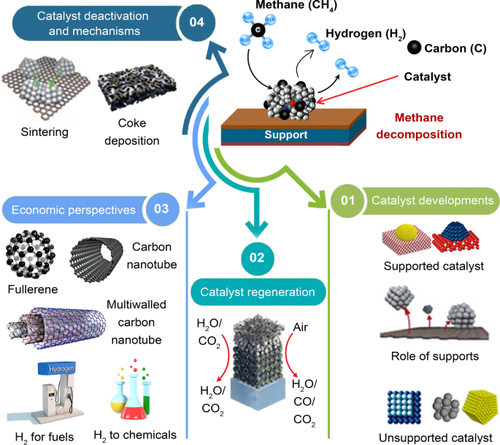
Carbon–neutral hydrogen production by catalytic methane decomposition: a review
The global hydrogen demand is projected to increase from 70 million tons in 2019 to more than 200 million tons in 2030. Methane decomposition is a promising reaction for H2 production, coupled with the synthesis of valuable carbon nanomaterials applicable in fuel cell technology, transportation fuels, and chemical synthesis. Here, we review catalytic methane decomposition, with focus on catalyst development, deactivation, reactivation, regeneration, and on economics. Catalysts include mono-, bi-, and trimetallic compounds and carbon-based compounds. Catalyst deactivation is induced by coke deposition. Despite remarkable strides in research, industrialization remains at an early stage.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Environmental Chemistry Letters
环境科学-工程:环境
CiteScore
32.00
自引率
7.00%
发文量
175
审稿时长
2 months
期刊介绍:
Environmental Chemistry Letters explores the intersections of geology, chemistry, physics, and biology. Published articles are of paramount importance to the examination of both natural and engineered environments. The journal features original and review articles of exceptional significance, encompassing topics such as the characterization of natural and impacted environments, the behavior, prevention, treatment, and control of mineral, organic, and radioactive pollutants. It also delves into interfacial studies involving diverse media like soil, sediment, water, air, organisms, and food. Additionally, the journal covers green chemistry, environmentally friendly synthetic pathways, alternative fuels, ecotoxicology, risk assessment, environmental processes and modeling, environmental technologies, remediation and control, and environmental analytical chemistry using biomolecular tools and tracers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


