用于刺激抗原递呈细胞中抗肿瘤先天免疫途径的合成阳离子螺旋多肽
IF 26.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
细胞内 DNA 传感器可调节先天性免疫,并为适应性免疫原性提供桥梁。然而,双链 DNA 或环状核苷酸等天然激动剂对抗原递呈细胞(APC)中传感器的激活作用因细胞内传递、血清稳定性、酶降解和快速全身清除等因素而受到阻碍。在这里,我们展示了螺旋多肽的疏水性、静电荷和次级构象可以优化,从而通过内质网应激刺激 APC 的先天性免疫通路。我们设计的三种多肽中的一种通过促进线粒体 DNA 的释放,优先激活了 APCs 中的两条主要细胞内 DNA 传感途径(cGAS-STING(环磷酸鸟苷-单磷酸腺苷合成酶-干扰素基因刺激器)和 Toll 样受体 9),从而高效地启动了效应 T 细胞。在局部晚期和转移性乳腺癌的合成小鼠模型中,多肽作为单一疗法或与免疫检查点抑制剂一起静脉注射,可产生有效的 DNA 传感器介导的抗肿瘤反应。通过工程阳离子多肽激活多种先天性免疫途径可能会为产生抗肿瘤免疫反应提供治疗优势。本文章由计算机程序翻译,如有差异,请以英文原文为准。
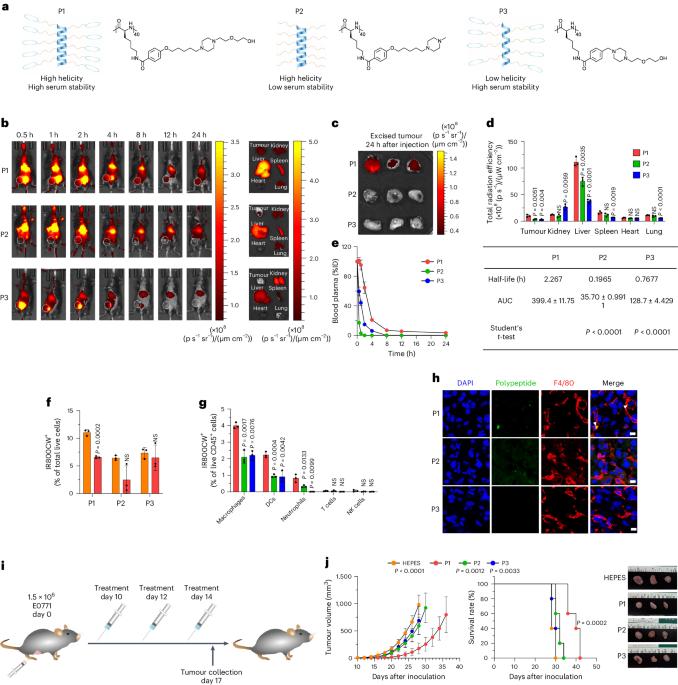
Synthetic cationic helical polypeptides for the stimulation of antitumour innate immune pathways in antigen-presenting cells
Intracellular DNA sensors regulate innate immunity and can provide a bridge to adaptive immunogenicity. However, the activation of the sensors in antigen-presenting cells (APCs) by natural agonists such as double-stranded DNAs or cyclic nucleotides is impeded by poor intracellular delivery, serum stability, enzymatic degradation and rapid systemic clearance. Here we show that the hydrophobicity, electrostatic charge and secondary conformation of helical polypeptides can be optimized to stimulate innate immune pathways via endoplasmic reticulum stress in APCs. One of the three polypeptides that we engineered activated two major intracellular DNA-sensing pathways (cGAS–STING (for cyclic guanosine monophosphate–adenosine monophosphate synthase–stimulator of interferon genes) and Toll-like receptor 9) preferentially in APCs by promoting the release of mitochondrial DNA, which led to the efficient priming of effector T cells. In syngeneic mouse models of locally advanced and metastatic breast cancers, the polypeptides led to potent DNA-sensor-mediated antitumour responses when intravenously given as monotherapy or with immune checkpoint inhibitors. The activation of multiple innate immune pathways via engineered cationic polypeptides may offer therapeutic advantages in the generation of antitumour immune responses. The hydrophobicity, electrostatic charge and secondary conformation of helical polypeptides can be optimized to stimulate antitumour innate immune responses via endoplasmic reticulum stress in antigen-presenting cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Biomedical Engineering
Medicine-Medicine (miscellaneous)
CiteScore
45.30
自引率
1.10%
发文量
138
期刊介绍:
Nature Biomedical Engineering is an online-only monthly journal that was launched in January 2017. It aims to publish original research, reviews, and commentary focusing on applied biomedicine and health technology. The journal targets a diverse audience, including life scientists who are involved in developing experimental or computational systems and methods to enhance our understanding of human physiology. It also covers biomedical researchers and engineers who are engaged in designing or optimizing therapies, assays, devices, or procedures for diagnosing or treating diseases. Additionally, clinicians, who make use of research outputs to evaluate patient health or administer therapy in various clinical settings and healthcare contexts, are also part of the target audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


