色丹内酯通过调节小鼠肠道 FXR-SMPD3 通路缓解 DSS 引起的结肠炎
IF 11.4
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
炎症性肠病(IBD)是一种治疗手段有限的全球性疾病。据报道,作为一种天然邻苯二甲酸酯,羟甲苯胺具有抗氧化和抗炎作用,但其对 IBD 的影响仍不清楚。在这项研究中,我们探讨了羟甲司坦内酯对右旋糖酐硫酸钠(DSS)诱导的小鼠结肠炎的影响。先给小鼠服用色丹内酯或载体,然后再给小鼠服用右旋糖酐硫酸钠,之后评估小鼠的结肠炎症状、炎症水平和肠道屏障功能。研究人员进行了转录组分析、16S rRNA测序以及胆汁酸和脂质的靶向代谢组学分析。色丹内酯能保护小鼠免受DSS诱导的结肠炎的影响,抑制炎症,恢复被削弱的上皮屏障,并通过减少胆盐水解酶(BSH)表达菌来改变肠道微生物群。色丹内酯对胆盐水解酶活性的下调增加了共轭胆汁酸(BA)与非共轭胆汁酸(BA)的比例,从而抑制了肠道法尼类固醇 X 受体(FXR)通路。我们使用肠道 FXR 特异性激动剂(非沙拉敏)和无菌小鼠分别验证了 FXR 通路和肠道微生物群的作用。此外,我们还发现了关键效应物质神经酰胺,它受鞘磷脂磷酸二酯酶 3 (SMPD3) 的调控。神经酰胺(d18:1/16:0)对炎症和肠道屏障的保护作用在体外使用人细胞系 Caco-2 得到了证实。Sedanolide能重塑肠道菌群并影响BA的组成,从而抑制FXR-SMPD3通路以刺激神经酰胺的合成,最终缓解DSS诱导的小鼠结肠炎。总之,我们的研究揭示了羟甲内酯对DSS诱导的小鼠结肠炎的保护作用,这表明羟甲内酯可作为结肠炎的临床治疗药物。此外,研究还发现关键脂质神经酰胺(d18:1/16:0)介导了羟基苯磺酸内酯的保护作用,这为人们了解结肠炎与脂质代谢物之间的关系提供了新的视角。本文章由计算机程序翻译,如有差异,请以英文原文为准。
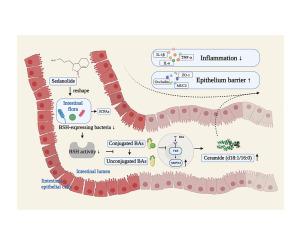

Sedanolide alleviates DSS-induced colitis by modulating the intestinal FXR-SMPD3 pathway in mice
Introduction
Inflammatory bowel disease (IBD) is a global disease with limited therapy. It is reported that sedanolide exerts anti-oxidative and anti-inflammatory effects as a natural phthalide, but its effects on IBD remain unclear.
Objectives
In this study, we investigated the impacts of sedanolide on dextran sodium sulfate (DSS)-induced colitis in mice.
Methods
The mice were administered sedanolide or vehicle followed by DSS administration, after which colitis symptoms, inflammation levels, and intestinal barrier function were evaluated. Transcriptome analysis, 16S rRNA sequencing, and targeted metabolomics analysis of bile acids and lipids were performed.
Results
Sedanolide protected mice from DSS-induced colitis, suppressed the inflammation, restored the weakened epithelial barrier, and modified the gut microbiota by decreasing bile salt hydrolase (BSH)-expressing bacteria. The downregulation of BSH activity by sedanolide increased the ratio of conjugated/unconjugated bile acids (BAs), thereby inhibiting the intestinal farnesoid X receptor (FXR) pathway. The roles of the FXR pathway and gut microbiota were verified using an intestinal FXR-specific agonist (fexaramine) and germ-free mice, respectively. Furthermore, we identified the key effector ceramide, which is regulated by sphingomyelin phosphodiesterase 3 (SMPD3). The protective effects of ceramide (d18:1/16:0) against inflammation and the gut barrier were demonstrated in vitro using the human cell line Caco-2.
Conclusion
Sedanolide could reshape the intestinal flora and influence BA composition, thus inhibiting the FXR-SMPD3 pathway to stimulate the synthesis of ceramide, which ultimately alleviated DSS-induced colitis in mice. Overall, our research revealed the protective effects of sedanolide against DSS-induced colitis in mice, which indicated that sedanolide may be a clinical treatment for colitis. Additionally, the key lipid ceramide (d18:1/16:0) was shown to mediate the protective effects of sedanolide, providing new insight into the associations between colitis and lipid metabolites.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


