DNA双链断裂捕获核膜小管驱动DNA修复
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
目前的模型表明,DNA 双链断裂(DSB)可以移动到核外围进行修复。目前还不清楚人类DSB在多大程度上表现出这种重新定位。在这里,我们发现人类核包膜定位到DSB的方式取决于DNA损伤应答(DDR)激酶和被α-tubulin乙酰转移酶-1(ATAT1)乙酰化的细胞质微管。这些因子与核骨架和细胞骨架复合体连接体(LINC)、核孔复合体(NPC)蛋白 NUP153、核薄层以及驱动蛋白 KIF5B 和 KIF13B 协作生成 DSB 捕捉核包膜小管(dsbNET)。虽然dsbNETs能促进修复和存活,但它们也会在多聚(ADP-核糖)聚合酶(PARP)抑制过程中被共同使用,以抑制BRCA1缺陷的乳腺癌细胞,并在表达与衰老相关的片层蛋白A突变体progerin的细胞中被过度诱导。总之,我们的研究结果促进了对核结构-功能关系的理解,发现了核-细胞质 DDR,并确定了 dsbNETs 是基因组组织和稳定性的关键因素。本文章由计算机程序翻译,如有差异,请以英文原文为准。
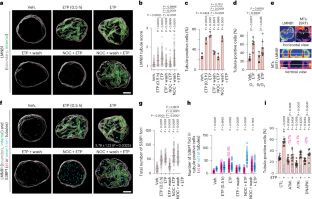
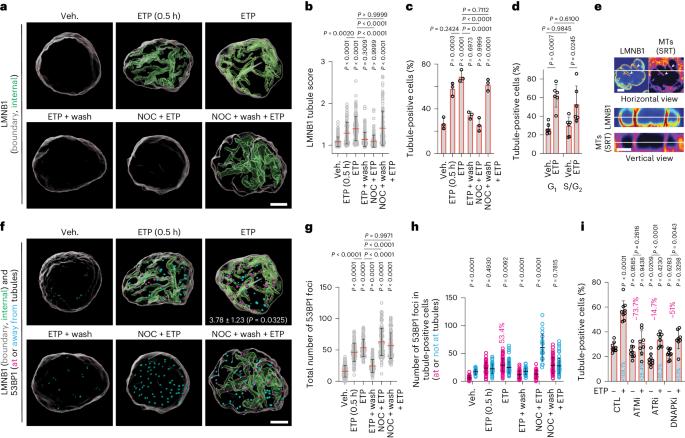
DNA double-strand break–capturing nuclear envelope tubules drive DNA repair
Current models suggest that DNA double-strand breaks (DSBs) can move to the nuclear periphery for repair. It is unclear to what extent human DSBs display such repositioning. Here we show that the human nuclear envelope localizes to DSBs in a manner depending on DNA damage response (DDR) kinases and cytoplasmic microtubules acetylated by α-tubulin acetyltransferase-1 (ATAT1). These factors collaborate with the linker of nucleoskeleton and cytoskeleton complex (LINC), nuclear pore complex (NPC) protein NUP153, nuclear lamina and kinesins KIF5B and KIF13B to generate DSB-capturing nuclear envelope tubules (dsbNETs). dsbNETs are partly supported by nuclear actin filaments and the circadian factor PER1 and reversed by kinesin KIFC3. Although dsbNETs promote repair and survival, they are also co-opted during poly(ADP-ribose) polymerase (PARP) inhibition to restrain BRCA1-deficient breast cancer cells and are hyper-induced in cells expressing the aging-linked lamin A mutant progerin. In summary, our results advance understanding of nuclear structure–function relationships, uncover a nuclear–cytoplasmic DDR and identify dsbNETs as critical factors in genome organization and stability. Here the authors show that the nucleus undergoes a transient ‘metamorphosis’ within a nuclear–cytoplasmic DNA damage response linked to health and disease. Through this process, the nuclear envelope projects tubules that capture damaged DNA, mediating its repair.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


