作为双齿卤素键阴离子受体的铱络合物具有更强的光学响应能力
IF 3.1
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
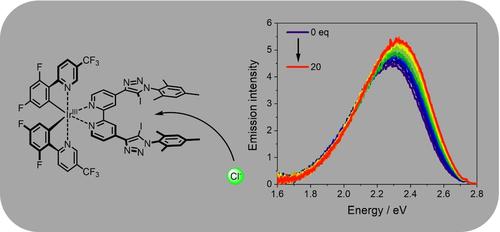
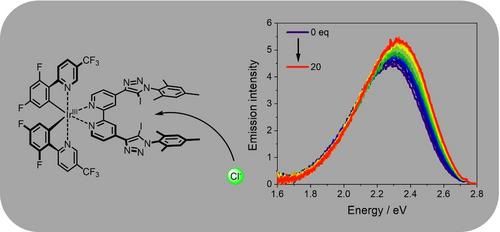
我们展示了一种发光铱(III)配合物,其特点是具有一个能与阴离子强结合的双叉卤素键供体位点。与之前基于 Ir(III) 的化学传感器(2.5%)相比,量身定制的 Ir(III)(L)2 分子的发射量子产率(8.4%)显著提高。利用发射滴定法证明了氯化物、溴化物和醋酸盐的成功结合。这些实验揭示了高达 1.6×105 M-1 的结合常数。此外,还首次采用了一种利用发射位移来评估结合常数的新方法。量子化学模拟支持实验观察到的特征。本文章由计算机程序翻译,如有差异,请以英文原文为准。
An Iridium Complex as Bidentate Halogen Bond-Based Anion Receptor Featuring an IncreasedOptical Response
We present a luminescent Ir(III) complex featuring a bidentate halogen bond donor site capable of strong anion binding. The tailor-made Ir(III)(L)2 moiety offers a significantly higher emission quantum yield (8.4 %) compared to previous Ir(III)-based chemo-sensors (2.5 %). The successful binding of chloride, bromide and acetate is demonstrated using emission titrations. These experiments reveal association constants of up to 1.6×105 M−1. Furthermore, a new approach to evaluate the association constant by utilizing the shift of the emission was used for the first time. The experimentally observed characteristics are supported by quantum chemical simulations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ChemistryOpen
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
4.80
自引率
4.30%
发文量
143
审稿时长
1 months
期刊介绍:
ChemistryOpen is a multidisciplinary, gold-road open-access, international forum for the publication of outstanding Reviews, Full Papers, and Communications from all areas of chemistry and related fields. It is co-owned by 16 continental European Chemical Societies, who have banded together in the alliance called ChemPubSoc Europe for the purpose of publishing high-quality journals in the field of chemistry and its border disciplines. As some of the governments of the countries represented in ChemPubSoc Europe have strongly recommended that the research conducted with their funding is freely accessible for all readers (Open Access), ChemPubSoc Europe was concerned that no journal for which the ethical standards were monitored by a chemical society was available for such papers. ChemistryOpen fills this gap.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


