植物防御素 MtDef4 的作用模式和作为多肽生物杀菌剂的潜力
IF 4.8
1区 农林科学
Q1 PLANT SCIENCES
引用次数: 0
摘要
由于植物真菌病原体对单位点杀真菌剂的抗药性迅速出现,对安全的多位点杀真菌剂的需求急剧增加。具有多位点作用模式(MoA)的植物抗真菌肽具有作为生物启发杀真菌剂的潜力。据报道,Medicago truncatula防御素MtDef4对真菌病原体具有很强的抗真菌活性。其 MoA 包括质膜破坏和与细胞内靶标结合。然而,这种防御素抑制并导致细胞死亡的具体生化过程尚未确定。在这里,我们发现 MtDef4 对灰霉病菌具有很强的抗真菌活性。它诱导该病原体的胚芽出现严重的质膜和细胞器不规则现象。它与真菌核糖体结合并抑制体外蛋白质翻译。缺乏抗真菌活性的 MtDef4 变体的蛋白质翻译抑制活性大大降低。生成的耐阳离子 MtDef4 变体与真菌细胞壁中的β-葡聚糖结合的亲和力高于 MtDef4。当在烟草植物、番茄果实和玫瑰花瓣上外源施用时,它比 MtDef4 更能减轻灰霉病症状。我们的研究结果揭示了抑制蛋白质合成可能是 MtDef4 的靶标,以及其耐受阳离子变体作为多肽类杀菌剂的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
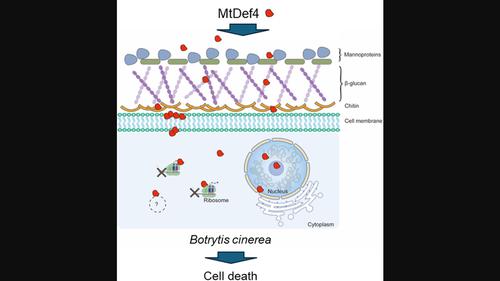
Modes of action and potential as a peptide‐based biofungicide of a plant defensin MtDef4
Due to rapidly emerging resistance to single‐site fungicides in fungal pathogens of plants, there is a burgeoning need for safe and multisite fungicides. Plant antifungal peptides with multisite modes of action (MoA) have potential as bioinspired fungicides. Medicago truncatula defensin MtDef4 was previously reported to exhibit potent antifungal activity against fungal pathogens. Its MoA involves plasma membrane disruption and binding to intracellular targets. However, specific biochemical processes inhibited by this defensin and causing cell death have not been determined. Here, we show that MtDef4 exhibited potent antifungal activity against Botrytis cinerea . It induced severe plasma membrane and organelle irregularities in the germlings of this pathogen. It bound to fungal ribosomes and inhibited protein translation in vitro. A MtDef4 variant lacking antifungal activity exhibited greatly reduced protein translation inhibitory activity. A cation‐tolerant MtDef4 variant was generated that bound to β‐glucan of the fungal cell wall with higher affinity than MtDef4. It also conferred a greater reduction in the grey mould disease symptoms than MtDef4 when applied exogenously on Nicotiana benthamiana plants, tomato fruits and rose petals. Our findings revealed inhibition of protein synthesis as a likely target of MtDef4 and the potential of its cation‐tolerant variant as a peptide‐based fungicide.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular plant pathology
生物-植物科学
CiteScore
9.40
自引率
4.10%
发文量
120
审稿时长
6-12 weeks
期刊介绍:
Molecular Plant Pathology is now an open access journal. Authors pay an article processing charge to publish in the journal and all articles will be freely available to anyone. BSPP members will be granted a 20% discount on article charges. The Editorial focus and policy of the journal has not be changed and the editorial team will continue to apply the same rigorous standards of peer review and acceptance criteria.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


