ASOptimizer:通过深度学习优化反义寡核苷酸,实现 IDO1 基因调控
IF 6.5
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
摘要
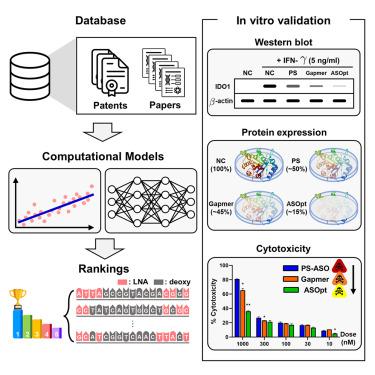
最近的研究突显了使用反义寡核苷酸(ASO)进行细胞 RNA 调控的有效性,包括那些被认为是不可药用的靶点;然而,人工设计最佳 ASO 序列可能会耗费大量人力和时间,这可能会限制其更广泛的应用。为了应对这一挑战,我们引入了一个平台--ASOptimizer,这是一个基于深度学习的框架,能以低成本高效设计 ASO。该平台不仅能选择最有效的 mRNA 靶点,还能优化化学修饰以提高性能。吲哚胺 2,3-二氧化酶 1(IDO1)通过消耗色氨酸和产生犬尿氨酸来促进癌症生存,从而在肿瘤微环境中通过 AhR 途径导致免疫抑制。我们使用 ASOptimizer 来识别以 IDO1 mRNA 为靶点的 ASO,将其作为潜在的癌症治疗药物。我们的方法包括两个阶段:序列工程和化学工程。在序列工程阶段,我们优化并预测了能有效靶向 IDO1 mRNA 的 ASO 序列。在化学工程阶段,我们进一步完善了这些 ASO,以增强其抑制活性,同时降低其潜在的细胞毒性。总之,我们的研究证明了 ASOptimizer 在鉴定具有更好疗效和安全性的 ASO 方面的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
ASOptimizer: Optimizing antisense oligonucleotides through deep learning for IDO1 gene regulation
Recent studies have highlighted the effectiveness of using antisense oligonucleotides (ASOs) for cellular RNA regulation, including targets that are considered undruggable; however, manually designing optimal ASO sequences can be labor intensive and time consuming, which potentially limits their broader application. To address this challenge, we introduce a platform, the ASOptimizer, a deep-learning-based framework that efficiently designs ASOs at a low cost. This platform not only selects the most efficient mRNA target sites but also optimizes the chemical modifications for enhanced performance. Indoleamine 2,3-dioxygenase 1 (IDO1) promotes cancer survival by depleting tryptophan and producing kynurenine, leading to immunosuppression through the AhR pathway within the tumor microenvironment. We used ASOptimizer to identify ASOs that target IDO1 mRNA as potential cancer therapeutics. Our methodology consists of two stages: sequence engineering and chemical engineering. During the sequence-engineering stage, we optimized and predicted ASO sequences that could target IDO1 mRNA efficiently. In the chemical-engineering stage, we further refined these ASOs to enhance their inhibitory activity while reducing their potential cytotoxicity. In conclusion, our research demonstrates the potential of ASOptimizer for identifying ASOs with improved efficacy and safety.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Therapy. Nucleic Acids
MEDICINE, RESEARCH & EXPERIMENTAL-
CiteScore
15.40
自引率
1.10%
发文量
336
审稿时长
20 weeks
期刊介绍:
Molecular Therapy Nucleic Acids is an international, open-access journal that publishes high-quality research in nucleic-acid-based therapeutics to treat and correct genetic and acquired diseases. It is the official journal of the American Society of Gene & Cell Therapy and is built upon the success of Molecular Therapy. The journal focuses on gene- and oligonucleotide-based therapies and publishes peer-reviewed research, reviews, and commentaries. Its impact factor for 2022 is 8.8. The subject areas covered include the development of therapeutics based on nucleic acids and their derivatives, vector development for RNA-based therapeutics delivery, utilization of gene-modifying agents like Zn finger nucleases and triplex-forming oligonucleotides, pre-clinical target validation, safety and efficacy studies, and clinical trials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


