用 CX3SO2Cl 进行催化剂和无碱可见光驱动的自由基中继三卤甲基化/官能团迁移/羰基化反应
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
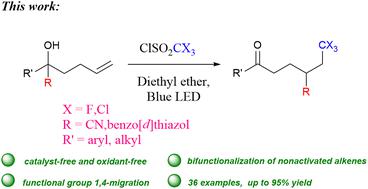
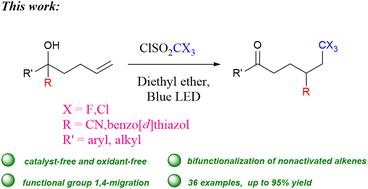
在此,我们报告了在没有光催化剂、过渡金属和碱的情况下,以 CX3SO2Cl 为 CX3 源(X = F、Cl),利用可见光使 2-羟基-2-己-5-烯腈发生自由基三卤甲基化/氰基迁移/羰基化级联反应,从而得到 5-氧代-2-(2,2,2-三卤乙基)戊腈化合物。该反应体系还能有效地将(苯并[d]噻唑-2-基)-戊-4-烯醇转化为相应的 4-(苯并[d]噻唑-2-基)-6,6,6-三卤代己酮产品。这些反应在温和的条件下进行,可容忍多种官能团,为未活化烯烃的 1,2-双官能化反应提供了替代方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Catalyst- and base-free visible light-enabled radical relay trihalomethylation/functional group-migration/carbonylation with CX3SO2Cl†
Herein, we report a visible light-enabled radical trihalomethylation/cyano-migration/carbonylation cascade reaction of 2-hydroxy-2-hex-5-enenitrile with CX3SO2Cl as the CX3-source (X = F, Cl) to obtain 5-oxo-2-(2,2,2-trihaloethyl)pentanenitrile compounds in the absence of a photocatalyst, transition metal and base. This reaction system is also effective to convert (benzo[d]thiazol-2-yl)-pent-4-enol to the corresponding 4-(benzo[d]thiazol-2-yl)-6,6,6-trihalo-hexanone products. These reactions occur under mild conditions, tolerate a wide range of functional groups, and provide alternative approaches for the 1,2-bifunctionalization reaction of unactivated olefins.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


