利用介质阻挡放电反应器进行二氧化碳氢化的等离子化学机制
IF 2.9
3区 物理与天体物理
Q2 PHYSICS, APPLIED
引用次数: 0
摘要
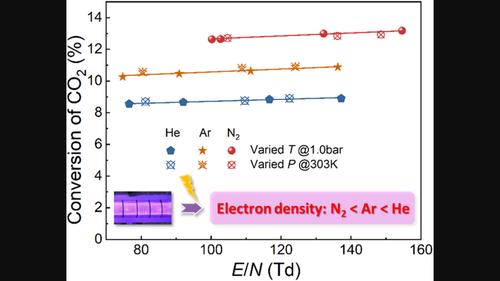
等离子体诱导的二氧化碳加氢过程已受到广泛关注,但相关的等离子体化学却未得到深入探讨。本文研究了在介质势垒放电反应器中二氧化碳加氢的电子诱导效应和热化学效应。CO2/H2、CO2/H2/N2、CO2/H2/Ar 和 CO2/H2/He 混合物的放电模式转变温度分别为 623、623、600 和 600 K。二氧化碳的转化受电子诱导反应控制,对放电模式和电子密度敏感,但对电子能量不敏感。相比之下,产物的形成受热诱导化学反应的控制。这些结果有助于更好地理解等离子体诱导的二氧化碳氢化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Mechanism of plasma chemistry in CO2 hydrogenation using a dielectric barrier discharge reactor
Plasma‐induced CO2 hydrogenation process has received much attention, while the related plasma chemistry has not been profoundly explored. Herein, electron‐induced and thermochemical effects on CO2 hydrogenation in a dielectric barrier discharge reactor were investigated. The temperatures for the discharge pattern transition for CO2 /H2 , CO2 /H2 /N2 , CO2 /H2 /Ar, and CO2 /H2 /He mixtures were 623, 623, 600, and 600 K, respectively. CO2 conversion was controlled by electron‐induced reactions and was sensitive to discharge pattern and electron density but not electron energy. In contrast, product formation was governed by the thermo‐induced chemistry. These results are useful for a better understanding of plasma‐induced CO2 hydrogenation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Plasma Processes and Polymers
物理-高分子科学
CiteScore
6.60
自引率
11.40%
发文量
150
审稿时长
3 months
期刊介绍:
Plasma Processes & Polymers focuses on the interdisciplinary field of low temperature plasma science, covering both experimental and theoretical aspects of fundamental and applied research in materials science, physics, chemistry and engineering in the area of plasma sources and plasma-based treatments.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


