二氧化碳反应性二元混合物的热学和物理学特性
IF 2
3区 工程技术
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
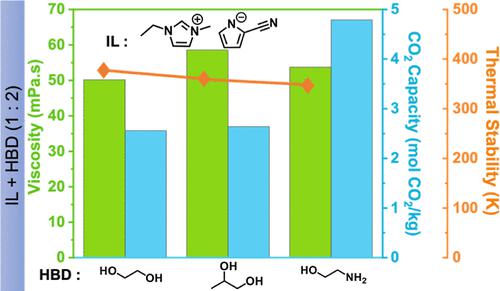
以离子液体(IL)1-乙基-3-甲基咪唑鎓 2-氰基吡咯内酯([EMIM][2-CNpyr])和一系列氢键给体(HBD)(包括乙二醇(EG)、丙二醇(PG)和单乙醇胺(MEA))为基础,对二元溶剂混合物的密度和粘度随温度变化的特性及其热稳定性和二氧化碳吸收-解吸能力进行了表征。利用核磁共振和傅立叶变换红外光谱观察了 IL/HBD 成分对分子间相互作用的影响。在所研究的混合物中,IL/EG(1:2)显示出最高效的吸收-解吸性能和热稳定性。虽然 IL/PG 和 IL/EG 具有相似的二氧化碳吸收能力,但 IL/PG 的粘度最高,限制了二氧化碳在溶剂中的传输。IL/MEA 溶剂具有显著的二氧化碳吸收能力,但 MEA 与二氧化碳之间的强结合能以及吸收过程中粘度的增加导致二氧化碳解吸困难。这项研究强调了 IL/HBD 二元混合物中分子间相互作用的改变与 HBD 的选择有关,这反映在它们不同的物理性质和二氧化碳结合行为上。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Thermal and Physical Properties of CO2-Reactive Binary Mixtures
Binary solvent mixtures based on 1-ethyl-3-methylimidazolium 2-cyanopyrrolide, [EMIM][2-CNpyr], an ionic liquid (IL), and a series of hydrogen bond donors (HBDs) including ethylene glycol (EG), propylene glycol (PG), and monoethanolamine (MEA) were characterized in terms of temperature-dependent densities and viscosities along with their thermal stability and CO2 absorption–desorption capability. NMR and FTIR were employed to observe the effect of the IL/HBD composition on intermolecular interactions. Among the investigated mixtures, IL/EG (1:2) showed the most efficient absorption–desorption performance and thermal stability. Though IL/PG and IL/EG had similar CO2 absorption capacities, the IL/PG exhibited the highest viscosity, which limited the CO2 transport in the solvent. The IL/MEA solvent possesses significant CO2 absorbance capability; however, the strong binding energy between MEA and CO2 and the increased viscosity during absorption led to difficulties in CO2 desorption. This study highlights the modification of intermolecular interactions in IL/HBD binary mixtures with respect to the choice of HBDs, reflected by their distinct physical properties and CO2 binding behavior.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Chemical & Engineering Data
工程技术-工程:化工
CiteScore
5.20
自引率
19.20%
发文量
324
审稿时长
2.2 months
期刊介绍:
The Journal of Chemical & Engineering Data is a monthly journal devoted to the publication of data obtained from both experiment and computation, which are viewed as complementary. It is the only American Chemical Society journal primarily concerned with articles containing data on the phase behavior and the physical, thermodynamic, and transport properties of well-defined materials, including complex mixtures of known compositions. While environmental and biological samples are of interest, their compositions must be known and reproducible. As a result, adsorption on natural product materials does not generally fit within the scope of Journal of Chemical & Engineering Data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


