细菌还原过程中紫外线的同位素特征
IF 3.7
1区 地球科学
Q1 GEOCHEMISTRY & GEOPHYSICS
引用次数: 0
摘要
在细菌将六氟化铀还原为四氟化铀的过程中,通常会伴随着与质量无关的 238U 和 235U 同位素的两步电子转移,即重同位素在还原产物中累积。然而,紫外线中间体在分馏机制中的作用却因其化学稳定性方面的挑战而悬而未决。在这里,我们利用紫外线稳定配体 dpaea2- 在一龄雪旺菌还原六亚甲基六烷基硫醚的过程中捕获水体中的紫外线。从紫外分光光度计到紫外分光光度计的第一个还原步骤显示出可忽略不计的分馏,而从紫外分光光度计到紫外分光光度计的还原则显示出质量依赖性同位素分馏(235U 优先还原),这与之前的大多数观察结果相反。这种令人惊讶的行为突显了铀配位体对反应物铀供应、电子传递速率和 UIV 产物螯合之间平衡的控制作用,表明在使用铀同位素比值重建环境氧化还原条件时应考虑紫外分馏。本文章由计算机程序翻译,如有差异,请以英文原文为准。
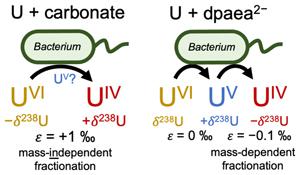
The isotopic signature of UV during bacterial reduction
The two step electron transfer during bacterial reduction of UVI to UIV is typically accompanied by mass-independent fractionation of the 238U and 235U isotopes, whereby the heavy isotope accumulates in the reduced product. However, the role of the UV intermediate in the fractionation mechanism is unresolved due to the challenges associated with its chemical stability. Here, we employed the UV stabilising ligand, dpaea2-, to trap aqueous UV during UVI reduction by Shewanella oneidensis. Whilst the first reduction step from UVI to UV displayed negligible fractionation, reduction of UV to UIV revealed mass-dependent isotope fractionation (preferential reduction of the 235U), contrary to most previous observations. This surprising behaviour highlights the control that the U-coordinating ligand exerts over the balance between reactant U supply, electron transfer rate, and UIV product sequestration, suggesting that UV speciation should be considered when using U isotope ratios to reconstruct environmental redox conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Geochemical Perspectives Letters
Earth and Planetary Sciences-Geochemistry and Petrology
CiteScore
7.00
自引率
2.00%
发文量
42
审稿时长
15 weeks
期刊介绍:
Geochemical Perspectives Letters is an open access, internationally peer-reviewed journal of the European Association of Geochemistry (EAG) that publishes short, highest-quality articles spanning geochemical sciences. The journal aims at rapid publication of the most novel research in geochemistry with a focus on outstanding quality, international importance, originality, and stimulating new developments across the vast array of geochemical disciplines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


