基于烷氧基噻吩侧翼苯并二噻唑的高结晶面π共轭聚合物,用于有机光伏技术
IF 2.3
4区 化学
Q3 POLYMER SCIENCE
引用次数: 0
摘要
利用非共价分子内相互作用是制备π-共轭聚合物的一种强有力的设计策略,这种聚合物具有很高的骨架共面性,因此结晶度也很高。在此,我们报告了烷氧基噻吩侧翼苯并二噻唑(BBTz)作为π-共轭聚合物新构建单元的设计与合成,随后将其共聚,得到了简单的带有烷基和烷氧基的BBTz-二噻吩共聚物(PDBTz2)。由于 BBTz 中的烷氧基氧和噻唑硫之间存在 S-O 非共价分子内相互作用,PDBTz2 比其烷基对应物 PDBTz1 显示出更高的共面性和结晶性。有趣的是,PDBTz2 的骨架取向从 PDBTz1 的边缘取向完全改变为正面取向,这对于有机光伏(OPV)来说更为理想。此外,与 PDBTz1 相比,烷氧基的电子捐献性质提高了 PDBTz2 的 HOMO 能级,从而使光诱导空穴从非富勒烯受体 Y6 转移到聚合物。因此,基于 PDBTz2 和 Y6 的有机光伏电池的短路电流密度明显高于基于 PDBTz1 和 Y6 的电池。这项研究证实,烷氧基噻吩侧翼 BBTz 是一种很有前景的高性能 π 共轭聚合物构建单元。本文章由计算机程序翻译,如有差异,请以英文原文为准。
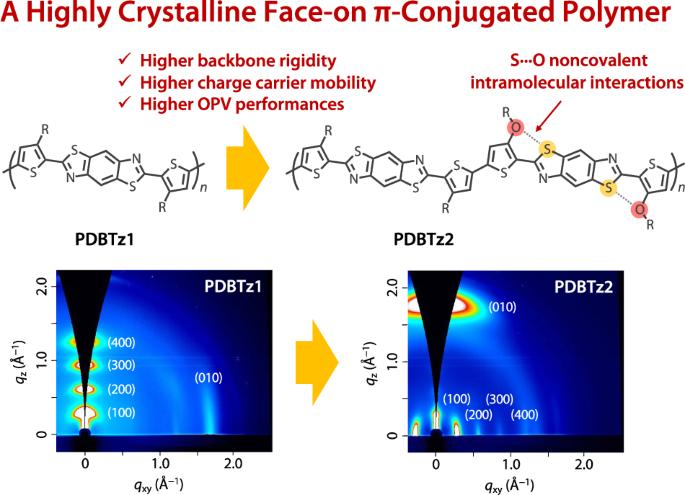
A highly crystalline face-on π-conjugated polymer based on alkoxythiophene-flanked benzobisthiazole for organic photovoltaics
The use of noncovalent intramolecular interactions constitutes a powerful design strategy for preparing π-conjugated polymers featuring high backbone coplanarities and thereby high crystallinities. Herein, we report the design and synthesis of an alkoxythiophene-flanked benzobisthiazole (BBTz) as a new building unit for π-conjugated polymers, which was subsequently copolymerized to give a simple BBTz-bithiophene copolymer with alkyl and alkoxy groups (PDBTz2). Owing to the S···O noncovalent intramolecular interactions between the alkoxy oxygens and thiazole sulfurs in BBTz, PDBTz2 showed greater coplanarity and crystallinity than its alkyl counterpart, PDBTz1. Interestingly, the backbone orientation was completely altered from the edge-on orientation observed for PDBTz1 to a face-on orientation for PDBTz2, which is preferable for organic photovoltaics (OPVs). In addition, the electron-donating nature of the alkoxy group increased the HOMO energy level of PDBTz2 compared to that of PDBTz1, which enabled photoinduced hole transfer from a nonfullerene acceptor, Y6, to the polymer. As a result, the short-circuit current density of an organic photovoltaic cell based on PDBTz2 and Y6 was significantly greater than that of a cell based on PDBTz1 and Y6. This study confirmed that alkoxythiophene-flanked BBTz is a promising building unit for high-performance π-conjugated polymers. We synthesized a new benzobisthiazole (BBTz) containing building unit in which two alkoxythiophenes were attached to the BBTz moiety so as to induce oxygen–sulfur noncovalent intramolecular interactions and thereby interlock the linkage. As a result, the π-conjugated polymer incorporating the new building unit, PDBTz2, had a more coplanar and rigid backbone than the alkyl counterpart, PDBTz1. Interestingly, the backbone orientation was completely altered from the edge-on orientation (PDBTz1) to the face-on orientation (PDBTz2), which is preferable for organic photovoltaics. Accordingly, PDBTz2 showed a much higher photovoltaic performance than PDBTz1.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Polymer Journal
化学-高分子科学
CiteScore
5.60
自引率
7.10%
发文量
131
审稿时长
2.5 months
期刊介绍:
Polymer Journal promotes research from all aspects of polymer science from anywhere in the world and aims to provide an integrated platform for scientific communication that assists the advancement of polymer science and related fields. The journal publishes Original Articles, Notes, Short Communications and Reviews.
Subject areas and topics of particular interest within the journal''s scope include, but are not limited to, those listed below:
Polymer synthesis and reactions
Polymer structures
Physical properties of polymers
Polymer surface and interfaces
Functional polymers
Supramolecular polymers
Self-assembled materials
Biopolymers and bio-related polymer materials
Polymer engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


