研究在氢能源中应用钡锶钴铁的可能性
摘要
Abstract 阴离子缺陷结构 based on ({text{S}}{{text{r}}}_{{0.5}}}{text{B}}{{text{a}}}_{0.5}}{{text{C}}{{{text{o}}_{{1 - x}}}{{text{F}}}{{{{text\{e}}}_{x}}}{{{{text{O}}}_{{3 - \delta }}}} (在大型太阳炉(LSF)中产生的密度为 100-200 W/cm2 的太阳集中辐射流中由熔体合成)进行了研究。根据碳酸盐和金属氧化物的化学计量混合物(\({\{SrC}}{{text{O}}}_{3}}\)+({text\{BrC}}{{text{O}}}_{3}}})制成的片状煤砖\({\{text{BaC}}{{text{O}}}_{3}}) + ({\{C}}{{text{o}}}_{2}}}{{{text{O}}}_{3}})在 LS 聚光器的水冷熔化单元中熔化。在 LSF 焦点区的水冷熔化装置中熔化。熔液滴入熔化装置下方 40 厘米处容器中的水中。这种条件有助于熔体以 103 K/s 的速度冷却。将铸件研磨至 63 微米的细度,在 673 K 下烘干,然后使用半干压(压力为 100 兆帕)将所得粉末制成直径为 20 毫米、高度为 10 毫米的片状样品。片剂在温度为 1050-1250°C 的空气中烧结。对成品样品的结构、吸水性和电性能进行了研究。该材料的晶格为包晶结构,单位晶胞参数为 A = 4.04 × \({{10}^{ - 10}}) m,空间群为 Рm3m。组成的均匀性面积为 \({\text{S}}{{\text{r}}}_{{0.5}}}{text{B}}{{{\text{a}}}_{{0.5}}{{text{C}}{{{text{o}}}_{{1-x}}}{text{F}}{{{text{e}}}_{x}}{{{text{O}}}_{{3-\delta }}}})对应的区间 x = [0; 0.7],其中 x 是引入元素的数量,而不是主要元素的数量。就结构和性能的稳定性而言,最理想的组成是({\text{S}}{{\text{r}}}_{{0.5}}}{\text{B}}{{{\text{a}}}_{{0.5}}}{\text{C}}{{{\text{o}}}_{{0.8}}}{\text{F}}{{{\text{e}}}_{{0.2}}}{{{\text{O}}}_{{2.78}}}\).所得材料的平均晶粒大小为 30-40 μm。晶粒主要呈球状和弯曲圆柱状。材料样品显示出很强的抗水蒸气能力。结构参数值表明,由 \({\text{S}}{{{text{r}}}_{{0.5}}}{{text{B}}{{{{text{a}}}_{{0.5}}}{{text{C}}}{{{text\{o}}}_{{0.8}}}{text{F}}{{text{e}}}_{{0.2}}}{{text{O}}}_{{2.78}}})可用作催化剂,通过甲烷的重整和氧化反应生成氢气和合成气。
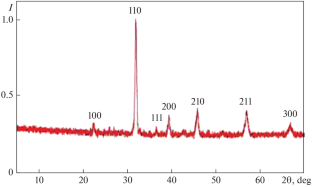

Anion-deficient structures based on \({\text{S}}{{{\text{r}}}_{{0.5}}}{\text{B}}{{{\text{a}}}_{{0.5}}}{\text{C}}{{{\text{o}}}_{{1 - x}}}{\text{F}}{{{\text{e}}}_{x}}{{{\text{O}}}_{{3 - \delta }}}\) synthesized from a melt in a stream of concentrated solar radiation with a density of 100–200 W/cm2 created in a large solar furnace (LSF) were studied. Briquettes in the form of tablets made on the basis of a stoichiometric mixture of carbonates and metal oxides (\({\text{SrC}}{{{\text{O}}}_{3}}\) + \({\text{BaC}}{{{\text{O}}}_{3}}\) + \({\text{C}}{{{\text{o}}}_{2}}{{{\text{O}}}_{3}}\) + \({\text{F}}{{{\text{e}}}_{2}}{{{\text{O}}}_{3}}\)) were melted in a water-cooled melting unit in the LSF focal zone. Drops of the melt flowed into the water in a container located 40 cm below the melting unit. Such conditions contributed to the cooling of the melt at a rate of 103 K/s. The castings were ground to a grinding fineness of 63 microns, dried at 673 K, and samples were molded from the resulting powder using semidry pressing (at a pressure of 100 MPa) in the form of tablets with a diameter of 20 mm and a height of 10 mm. The tablets were sintered in air at a temperature of 1050–1250°C. The structure, water absorption, and electrical properties of the finished samples were studied. The crystal lattice of the material had a perovskite structure with a unit cell parameter A = 4.04 × \({{10}^{{ - 10}}}\) m of space group Рm3m. The area of homogeneity of compositions \({\text{S}}{{{\text{r}}}_{{0.5}}}{\text{B}}{{{\text{a}}}_{{0.5}}}{\text{C}}{{{\text{o}}}_{{1 - x}}}{\text{F}}{{{\text{e}}}_{x}}{{{\text{O}}}_{{3 - \delta }}}\) corresponded to the interval x = [0; 0.7], where x is the amount of element introduced instead of the main one. The most optimal composition in terms of stability of structure and properties was \({\text{S}}{{{\text{r}}}_{{0.5}}}{\text{B}}{{{\text{a}}}_{{0.5}}}{\text{C}}{{{\text{o}}}_{{0.8}}}{\text{F}}{{{\text{e}}}_{{0.2}}}{{{\text{O}}}_{{2.78}}}\). The average crystallite size of the obtained materials is 30–40 μm. The grains are predominantly in the form of spherulites and curved cylinders. Samples of the material showed high resistance to water vapor. The values of structural parameters indicate that the material made from \({\text{S}}{{{\text{r}}}_{{0.5}}}{\text{B}}{{{\text{a}}}_{{0.5}}}{\text{C}}{{{\text{o}}}_{{0.8}}}{\text{F}}{{{\text{e}}}_{{0.2}}}{{{\text{O}}}_{{2.78}}}\) can be used as a catalyst in the generation of hydrogen and synthesis gas through reforming and oxidation of methane.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


