$${text{Mo}}{{text{O}}}_{{text\{2}}}}\{text{Cl}}_{{2\left( {{text{aq}}} \right)}}^{^\circ }$ 在 100-350°C 和饱和蒸汽压下的水热解稳定性的实验研究
IF 0.7
4区 地球科学
Q4 GEOCHEMISTRY & GEOPHYSICS
引用次数: 0
摘要
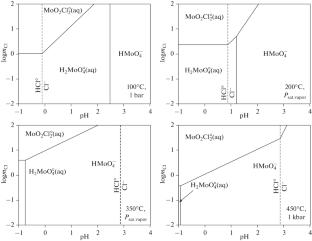
摘要 在 100、155、200、250、300、350°C 和饱和蒸汽压条件下,研究了晶体 MoO3 在不同浓度盐酸溶液中的溶解度。结果表明,随着盐酸浓度的增加,MoO3 的溶解度也在增加。使用 OptimA 程序测定了 MoO2Cl2 复合物的吉布斯能。根据反应计算出了 MoO2Cl2 的稳定常数:$${text{Mo}}{{text{O}}}_{{{text{3(c)}}}}}+ 2{\text{HC}}{{{\text{l}}}_{{{\text{(aq)}}}}}\to {\text{Mo}}{{{\text{O}}}_{{\text{2}}}}{\text{Cl}}_{{2{\text{(aq)}}}}^{^\circ }+ {{{text{H}}_{{text{2}}}}{{{{text{O}}}_{{{{text{(l)}}}}}.$$ 在 100、155、200、250、300、350°C(饱和蒸汽压)下,pK 值分别为 1.07 ± 0.29;1.06 ± 0.49;1.74 ± 0.71;1.83 ± 0.47;1.50 ± 0.28;0.95 ± 0.57。)本文章由计算机程序翻译,如有差异,请以英文原文为准。
Experimental Study of the \({\text{Mo}}{{{\text{O}}}_{{\text{2}}}}{\text{Cl}}_{{2\left( {{\text{aq}}} \right)}}^{^\circ }\) Stability in Hydrothermal Solutions at 100–350°C and Saturated Vapor Pressure
The solubility of crystalline MoO3 in HCl solutions with variable concentration was investigated at 100, 155, 200, 250, 300, 350°C and saturated vapor pressure. The results showed that the MoO3 solubility increases with increasing HCl concentration. Using the OptimA program, the Gibbs energies of MoO2Cl2 complex have been determined. The stability constants of MoO2Cl2 are calculated according to the reaction:
$${\text{Mo}}{{{\text{O}}}_{{{\text{3(c)}}}}} + 2{\text{HC}}{{{\text{l}}}_{{{\text{(aq)}}}}} \to {\text{Mo}}{{{\text{O}}}_{{\text{2}}}}{\text{Cl}}_{{2{\text{(aq)}}}}^{^\circ } + {{{\text{H}}}_{{\text{2}}}}{{{\text{O}}}_{{{\text{(l)}}}}}.$$
The pK values are 1.07 ± 0.29; 1.06 ± 0.49; 1.74 ± 0.71; 1.83 ± 0.47; 1.50 ± 0.28; 0.95 ± 0.57, respectively, at 100, 155, 200, 250, 300, 350°C (saturated vapor pressure).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Geochemistry International
地学-地球化学与地球物理
CiteScore
1.60
自引率
12.50%
发文量
89
审稿时长
1 months
期刊介绍:
Geochemistry International is a peer reviewed journal that publishes articles on cosmochemistry; geochemistry of magmatic, metamorphic, hydrothermal, and sedimentary processes; isotope geochemistry; organic geochemistry; applied geochemistry; and chemistry of the environment. Geochemistry International provides readers with a unique opportunity to refine their understanding of the geology of the vast territory of the Eurasian continent. The journal welcomes manuscripts from all countries in the English or Russian language.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


