针对 RAS 驱动型癌症的组合策略
IF 72.5
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
尽管 RAS 以前被认为是不可药用的,但现在已经开发出了各种抑制 RAS 或特定 RAS 癌蛋白的药物。事实上,最近突变选择性 KRAS 抑制剂在临床上取得的成功说明了直接靶向 RAS 的重要性。然而,对这些药物的反应通常是不完全的,而且仅限于一部分患者,这就凸显了开发更有效治疗方法的必要性,而这很可能需要一种组合方法。目前正在研究以 RAS 通路中的多个节点为靶点的纵向策略,以实现更深层次的抑制,这种策略在其他情况下已有先例。不过,针对 RAS 和其他治疗弱点的替代策略已经确定,这可能会减轻对深入抑制通路的要求。无论如何,任何特定方法的疗效都可能取决于遗传、表观遗传和肿瘤特异性变量。在此,我们讨论了治疗 KRAS 驱动的癌症的各种组合策略,并强调了可能扩展到携带其他 RAS 突变的肿瘤的机理概念。虽然已经发现了许多有前景的组合,但临床反应最终将取决于能否实现治疗窗口期,以及我们是否有能力前瞻性地选择有反应的患者。因此,我们必须继续开发和了解生物多样性策略,以最大限度地提高成功的可能性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
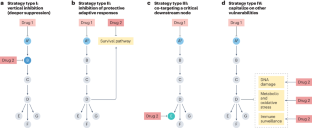
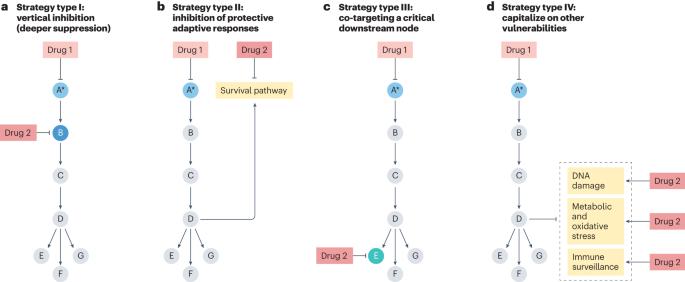
Combinatorial strategies to target RAS-driven cancers
Although RAS was formerly considered undruggable, various agents that inhibit RAS or specific RAS oncoproteins have now been developed. Indeed, the importance of directly targeting RAS has recently been illustrated by the clinical success of mutant-selective KRAS inhibitors. Nevertheless, responses to these agents are typically incomplete and restricted to a subset of patients, highlighting the need to develop more effective treatments, which will likely require a combinatorial approach. Vertical strategies that target multiple nodes within the RAS pathway to achieve deeper suppression are being investigated and have precedence in other contexts. However, alternative strategies that co-target RAS and other therapeutic vulnerabilities have been identified, which may mitigate the requirement for profound pathway suppression. Regardless, the efficacy of any given approach will likely be dictated by genetic, epigenetic and tumour-specific variables. Here we discuss various combinatorial strategies to treat KRAS-driven cancers, highlighting mechanistic concepts that may extend to tumours harbouring other RAS mutations. Although many promising combinations have been identified, clinical responses will ultimately depend on whether a therapeutic window can be achieved and our ability to prospectively select responsive patients. Therefore, we must continue to develop and understand biologically diverse strategies to maximize our likelihood of success. In this Review, Cichowski and colleagues provide an overview of combinatorial strategies designed to treat RAS-driven cancers that are based on four concepts that include vertical pathway inhibition, co-targeting RAS and adaptive survival pathways, co-targeting downstream or converging pathways and capitalizing on other cancer-associated vulnerabilities.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Reviews Cancer
医学-肿瘤学
CiteScore
111.90
自引率
0.40%
发文量
97
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Cancer, a part of the Nature Reviews portfolio of journals, aims to be the premier source of reviews and commentaries for the scientific communities it serves. The correct abbreviation for abstracting and indexing purposes is Nat. Rev. Cancer. The international standard serial numbers (ISSN) for Nature Reviews Cancer are 1474-175X (print) and 1474-1768 (online). Unlike other journals, Nature Reviews Cancer does not have an external editorial board. Instead, all editorial decisions are made by a team of full-time professional editors who are PhD-level scientists. The journal publishes Research Highlights, Comments, Reviews, and Perspectives relevant to cancer researchers, ensuring that the articles reach the widest possible audience due to their broad scope.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


