膜间空间定位的 TbTim15 是锥虫单线粒体内膜蛋白质转运酶的一个重要亚基
IF 2.6
2区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
所有线粒体都从细胞质中输入 95% 的蛋白质。这一过程由线粒体膜上的蛋白质转运酶介导,其亚基通常高度保守。大多数真核生物都有两种内膜蛋白转运酶(TIMs),专门用于导入含前序蛋白或线粒体载体蛋白。相比之下,寄生原生动物布氏锥虫只有一个由一个保守亚基和五个独特亚基组成的 TIM 复合物。在这里,我们利用先前表征过的 TIM 和 PAM 亚基的蛋白质-蛋白质相互作用网络,确定了 TIM 或前序列转运酶相关马达(PAM)新亚基的候选者。这项分析表明,锥虫 TIM 复合物包含一个额外的锥虫特异性亚基,命名为 TbTim15。TbTim15 与 TIM 复合物相关,缺乏跨膜结构域,并定位在膜间空间。TbTim15 对原环和血流形式的锥虫至关重要。它含有两个孪生 CX9C 基序,介导含前序和线粒体载体蛋白的导入。虽然 TbTim15 在线粒体蛋白导入中的确切功能尚不清楚,但我们的研究结果与它可能充当非典型锥虫 TIM 复合物的导入受体这一观点是一致的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
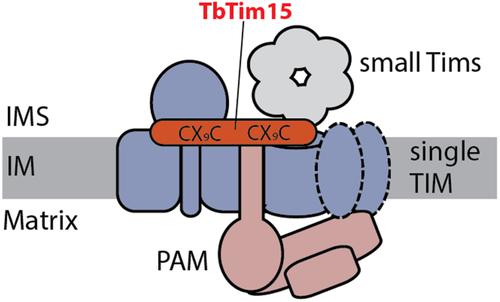
Intermembrane space-localized TbTim15 is an essential subunit of the single mitochondrial inner membrane protein translocase of trypanosomes
All mitochondria import >95% of their proteins from the cytosol. This process is mediated by protein translocases in the mitochondrial membranes, whose subunits are generally highly conserved. Most eukaryotes have two inner membrane protein translocases (TIMs) that are specialized to import either presequence-containing or mitochondrial carrier proteins. In contrast, the parasitic protozoan Trypanosoma brucei has a single TIM complex consisting of one conserved and five unique subunits. Here, we identify candidates for new subunits of the TIM or the presequence translocase-associated motor (PAM) using a protein–protein interaction network of previously characterized TIM and PAM subunits. This analysis reveals that the trypanosomal TIM complex contains an additional trypanosomatid-specific subunit, designated TbTim15. TbTim15 is associated with the TIM complex, lacks transmembrane domains, and localizes to the intermembrane space. TbTim15 is essential for procyclic and bloodstream forms of trypanosomes. It contains two twin CX9C motifs and mediates import of both presequence-containing and mitochondrial carrier proteins. While the precise function of TbTim15 in mitochondrial protein import is unknown, our results are consistent with the notion that it may function as an import receptor for the non-canonical trypanosomal TIM complex.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Microbiology
生物-生化与分子生物学
CiteScore
7.20
自引率
5.60%
发文量
132
审稿时长
1.7 months
期刊介绍:
Molecular Microbiology, the leading primary journal in the microbial sciences, publishes molecular studies of Bacteria, Archaea, eukaryotic microorganisms, and their viruses.
Research papers should lead to a deeper understanding of the molecular principles underlying basic physiological processes or mechanisms. Appropriate topics include gene expression and regulation, pathogenicity and virulence, physiology and metabolism, synthesis of macromolecules (proteins, nucleic acids, lipids, polysaccharides, etc), cell biology and subcellular organization, membrane biogenesis and function, traffic and transport, cell-cell communication and signalling pathways, evolution and gene transfer. Articles focused on host responses (cellular or immunological) to pathogens or on microbial ecology should be directed to our sister journals Cellular Microbiology and Environmental Microbiology, respectively.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


